Orthostatic Hypotension
Definition
Orthostatic or postural hypotension (OH) was first described by Bradbury and Eggleston in 1925.1 OH is diagnosed when there is a reduction of ≥20 mmHg in SBP or ≥10 mmHg in DBP within 3 min of standing or using an upright tilt table at an angle of at least 60° with or without symptoms.2 Although the definition is based on a consensus statement and the figures are arbitrary, a more modest drop in BP associated with symptoms is equally important. Delayed orthostatic hypotension (DOH), seen in 54% of patients with OH, is defined as a sustained fall in BP occurring beyond 3 min of standing or an upright tilt table test.3 Initial orthostatic hypotension (IOH) is defined as a transient decrease of ≥40 mmHg SBP and/or ≥20 mmHg DBP within 15 s after standing and is associated with symptoms of cerebral hypoperfusion.4
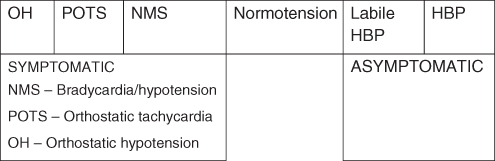
OH represents one end of the spectrum of disorders of cardiovascular dysregulation. The spectrum (Figure 39.1) extends from very low to very high BP. The majority of individuals with normal BP are in the middle. At the right end are hypertensive individuals who have elevated BP all the time. Labile hypertensive individuals with BP ranging from 120/80 to 140/90 occupy the borderland between hypertensive and normal population. Individuals on the right side are asymptomatic and are treated to prevent complications in the future. At the left extreme are the individuals with OH. Individuals with ‘mild dysautonomias’ span the region between the OH and normotensive groups and include people with postural tachycardia syndrome (POTS) and neurally mediated syncope (NMS). Individuals with POTS have orthostatic tachycardia whereas those with NMS have normal pressures in all postures but occasionally have ‘fainting’ associated with a brief period (usually less than 1 min) of hypotension and/or bradycardia. Individuals on the left side have symptoms that affect quality of life and could be dangerous.5
Figure 39.1 Cardiovascular dysregulation. HBP, high blood pressure; NMS, neurally mediated syncope; OH, orthostatic hypotension; POTS, postural tachycardia syndrome. Reproduced with permission from Robertson.5
Epidemiology
OH is a transient phenomenon with a high degree of intra-individual and intra-observer variability. OH is common among elderly populations with a varied prevalence of 6–34 % in community-dwelling people over 65 years of age6–9 and around 20% among ambulatory nursing home residents.10 Difference in measurement techniques of BP and timing of the measurement after change in position may contribute to the wide variations noted. The prevalence of OH increases with age from 14.8% in subjects aged 65–69 years to 26% in those 85 years and older. Differences in racial distribution have been documented by some, with predominance among whites,11 whereas others documented no such difference.12 The incidence of hospitalization secondary to OH increases with age and peaks over the age of 75 years at 233 per 100 000 patients. The median hospital stay is 3 days and the mortality rate is 0.9%.13 OH is an independent predictor of 4 year all-cause mortality, with an age-adjusted relative risk of 1.8 [95% confidence interval (CI), 1.22–2.65].14
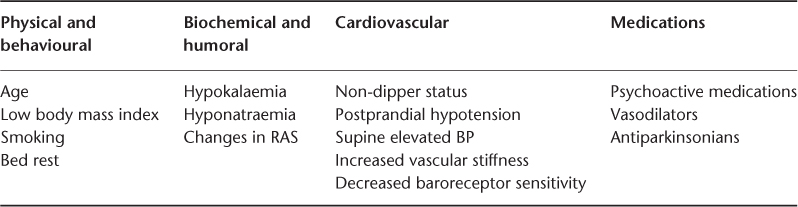
Many drugs have been implicated in either inducing or worsening of OH. These include antidepressants (tricyclic antidepressants, older monoamine oxidase inhibitors, serotonin–norepinephrine reuptake inhibitors), antipsychotics (phenothiazines), antihypertensives including diuretics,15 narcotics and alcohol. Many factors have been linked with increased risk of OH (Table 39.1).
Table 39.1 Factors linked with increased risk of orthostatic hypotension. Reproduced with permission from Hajjar.16.
OH is highly prevalent in patients with Parkinson’s disease (47%; range, 16–58%). Other neurological diseases with a high prevalence are pure autonomic failure (PAF) (33%), multiple system atrophy (MSA) (26%), idiopathic (autoimmune autonomic neuropathy) (17%) and diabetic autonomic neuropathy (14%). Among the diabetics, although the incidence of autonomic dysfunction is high (54% in type 1 and 73% in type 2), the prevalence of OH is not proportionate (8.4% in type 1 and 7.4% in type 2). OH increases the risk for coronary artery disease and all-cause mortality17 and is associated with systolic hypertension and low body mass index,18 stroke19 and chronic kidney disease.20
Mechanism
The adoption of an upright posture by humans posed challenges for the BP regulatory system and through evolution the body developed mechanisms to accommodate the effects of gravity- and activity-mediated fluid shifts. Hormonal factors such as the renin–angiotensin–aldosterone system regulate BP over long periods. Cardiovascular regulation by the autonomic nervous system (ANS) prevents more than a 5–10 mmHg drop in SBP, increases DBP and increases the pulse rate by 10–25 beats per minute (bpm). Sympathetic autonomic dysfunction can result in the development of OH.
In normal individuals, upon standing, roughly 500–800 ml of blood is displaced from the upper part of body to the lower part, primarily to the abdomen and lower extremities. The drop in volume (∼30%) reduces the venous return to the heart, leading to a drop in stroke volume and arterial pressure. This causes activation of two sets of pressure receptors: (a) high-pressure centres in the aortic arch and carotid sinuses and (b) low-pressure receptors in the heart and lungs. These receptors, present in both the atrium and the ventricles of the heart, produce a tonic inhibitory effect on the sympathoexcitary neurons in cardiovascular areas of the medulla. A fall in venous return diminishes the stretch, decreasing their firing rates, thus resulting in increased sympathetic outflow. This causes a rise in BP by increasing systemic vascular resistance and constriction of splanchnic capacitance vessels. The baroreceptors located in the carotid sinus at the origin of the internal carotid artery transmit the local stretch signals to the nucleus tractus solitarius along the glossopharyngeal nerve. These receptors are responsible for an immediate increase in heart rate in response to the drop in carotid arterial pressure that occurs during an upright tilt test.5 With ageing, the baroreceptor sensitivity and cardiovascular response to sympathetic stimulation are reduced, predisposing to OH.14
Prolonged orthostatic stress (20–30 min of standing) causes a substantial (20% in healthy adults) transcapillary filtration of the fluid shift from the blood into the interstitial space and causes additional peripheral pooling, thus decreasing venous return to the heart with a subsequent decline in BP and cardiac output. A progressive and sustained increase in muscle sympathetic nerve activity in response to prolonged orthostatic stress, together with the renin–angiotensin–aldosterone system, release of vasopressin and attenuation of atrial natriuretic factor, maintain cardiovascular homeostasis in the upright posture. This delayed OH is a consequence of one or more of the following: (a) increased peripheral venous pooling, (b) increased fluid transudation or (c) failure of the neural and humoral mechanisms.
Initial orthostatic hypotension (IOH) differs from typical OH in being transient, occurring immediately (within 15 s) upon standing, and is associated with a much greater fall in BP (≥40 mmHg SBP and/or ≥20 mmHg DBP). This can be documented only by continuous beat-to-beat BP monitoring during active standing, hence passive tilt is of no diagnostic value. IOH is thought to be due to a mismatch between cardiac output and vascular resistance. The sudden contraction of the muscles in both the legs and the abdomen produces a compression of resistance and capacitance vessels and together with the local venoarteriolar axon reflex that constricts flow to skin muscle and adipose tissue increases the peripheral vascular resistance, thus causing an initial increase in venous return. The increase in right atrial pressure produces reflex-mediated lowering of the BP. Overcompensation drops BP to the orthostatic range. IOH explains the transient symptoms of cerebral hypoperfusion that develops after waking from an overnight sleep.4
Symptoms of OH occur more commonly in the mornings and after meals and is worsened by a hot bath or shower; sudden postural change, fever and alcohol consumption. It may be provoked by exercise, coughing, straining to defecate and hyperventilation. Symptoms are dependent not only on the absolute fall in BP but also on the rate of change and the ability of the cardiovasculature to autoregulate. Symptoms range from light headedness to syncope and include dizziness, weakness, blurry vision, neck pain, headache, angina, disturbed speech, confusion, impaired cognition, fall and syncope. OH is often one aspect of a more generalized disturbance in cardiovascular regulation. In the initial phase, patients tolerate the symptoms as the BP rises during the day. With progression of dysregulation, patients exhibit erratic swings of BP in response to various physiological and pharmacological stresses and develop supine hypertension by the end of the day which can cause nocturnal polyuria by pressure natriuresis.21, 22
Causes of OH are broadly classified into acute and chronic (Table 39.2). Acute OH develops over a short duration, is more symptomatic and results from acute processes such as sepsis, dehydration or myocardial ischaemia. Chronic OH develops over a longer duration, is usually asymptomatic initially and is mostly secondary to central or peripheral nervous system diseases.22
Table 39.2 Classification of orthostatic hypotension.
| Acute | Chronic | |
| Neurogenic | Non-neurogenic | |
| Acute pandysautonomia | Ageing | |
| Acute paraneoplastic autonomic neuropathy | Hypertension | |
| Autoimmune autonomic ganglionopathy (AAG) | ||
| Bezold–Jarisch reflex activation | Central nervous system | |
| Botulism | Lewy body dementia | |
| Carotid sinus syncope | Multiple sclerosis | |
| Drug induced/toxic acute autonomic neuropathy | Multiple system atrophy (MSA) | |
| Guillain–Barré syndrome | Myelopathy | |
| Micturition syncope | Olivo-ponto-cerebellar atrophy | |
| Porphyria | Parkinson’s disease | |
| Posterior fossa tumours | ||
| Non-neurogenic | Spinal cord tumours | |
| Adrenal crisis | Diarrhoea | Strokes |
| Anaemia | Haemorrhage | Subacute combined degeneration |
| Arrhythmias | Mastocytosis | Syringomyelia |
| Arteriovenous malformation | Myocardial infarction | Transverse myelitis |
| Pheochromocytoma | ||
| Burns | Pregnancy | Peripheral nervous system |
| Carcinoid | Sepsis | Alcoholic polyneuropathy |
| Carditis | Vomiting | Amyloidosis |
| Congestive heart failure Dialysis | Autoimmune autonomic neuropathy | |
| Diabetes mellitus | ||
| Dopamine-b-hydroxylase deficiency | ||
| Drugs | Familial dysautonomia (Riley–Day syndrome) | |
| ACE inhibitors | Insulin | HIV/AIDS |
| Alpha receptor blockers | Marijuana | |
| Barbiturates | Monoamine oxidase inhibitors | Nutritional deficiency (vitamin B12, folate) |
| Beta-blockers | Nitrates | Paraneoplastic syndrome |
| Bromocriptine | Opiates | Pure autonomic failure (PAF) |
| Calcium-channel blockers | Phenothiazines | Tabes dorsalis |
| Diuretics | Sildenafil | Uraemia |
| Ethanol | Tricyclic antidepressants | Wernicke–Korsakoff syndrome |
| Hydralazine | Tizanidine | |
| Vincristine | ||
Evaluation and Diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree