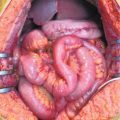
Fig. 10.1
Hyperthermic intraperitoneal chemotherapy (HIPEC): peritoneal cavity expander. (Courtesy of Prof. Angelo Di Giorgio)
A roller pump forces chemotherapy perfusion into the abdomen through the Tenckhoff catheter and extracts it through the drains, with a flow rate ∼1 L/min. A heat exchanger keeps the infused fluid at 43–45°C so that the intraperitoneal fluid is maintained at 41–43°C.
The perfusate is first recirculated between the reservoir and the heat exchanger so it can be heated to an adequate temperature. At this point, full circulation of the perfusate in and out of the peritoneal cavity is established until a minimum intraperitoneal temperature of 41.5°C is achieved and maintained. The drug is then added to the circuit, at which stage the perfusion timer is started.
In centers in which bidirectional chemotherapy protocols are used, intravenous infusion of the appropriate drugs is started synchronously with peritoneal chemotherapeutic infusion, although some surgeons prefer to start it 1 h before the actual peritoneal therapy.
Use of the coliseum technique was identified by Elias et al. [7] as the best technique in terms of thermal homogeneity and spatial diffusion. Those benefits are due to the ability to manipulate the intra-abdominal viscera during perfusion, which allows homogeneous exposition of all peritoneal surfaces to the therapy. Furthermore, as excessive heating of normal tissue is associated with a more lasting postoperative ileus and increases the incidence of postoperative perforation or fistula formation, this technique theoretically avoids these complications. The disadvantages of open HIPEC are heat dissipation to the surface of the perfusate, which makes it more difficult to achieve hyperthermia, and possible increased exposure of operative personnel to the chemotherapeutic agent, even if this is as yet only a theoretical risk.
10.4.2 Closed-abdomen Technique
Basically, this technique differs from the open technique only because the skin is sutured following laparotomy so that perfusion is done in a closed, watertight circuit (Fig. 10.2). Patient position varies during perfusion, which is achieved by tilting the surgical table into a Trendelenburg or anti- Trendelenburg position and then laterally in an attempt to promote uniform heat distribution. A larger volume of perfusate is generally needed to establish the circuit compared with during the open technique, and a higher abdominal pressure is achieved during perfusion that, as noted by Jacquet et al. [8], may facilitate drug penetration into tissue.
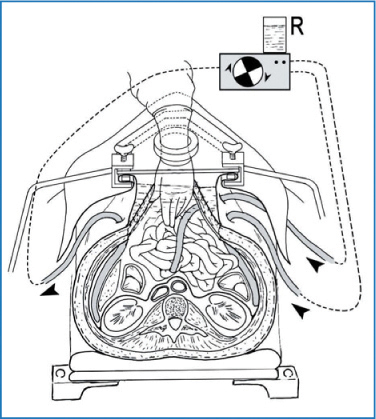

Fig. 10.2
Hyperthermic intraperitoneal chemotherapy (HIPEC): open technique. (Courtesy of Prof. Angelo Di Giorgio)
After hyperthermic perfusion, the abdomen is reopened and anastomoses, stoma, and drain placement are performed. The abdomen is then closed definitively in a standard manner. Otherwise, even when the closed technique is used, anastomoses and stoma are performed before abdominal wall closure. This way, there is no need to reopen the abdomen at the end of HIPEC, and catheters used for perfusion are used as drains during postoperative care.
The major advantage of the closed technique is the rapid achievement and constant maintenance of hyperthermia due to minimal heat loss. Moreover, exposure of OR personnel to aerosolized particles and contact with chemotherapeutic agents is minimized.
The lack of uniform distribution of the heated intraperitoneal chemotherapeutic agent is the main disadvantage of closed HIPEC. In fact, Elias et al. [7] reported an uneven distribution of methylene blue after its instillation during the procedure. Theoretically, inadequate circulation of heated intraperitoneal perfusate leads to pooling and accumulation of heat and the chemotherapeutic agent in the lower part of the body. This may result in increased systemic absorption and focal hyperthermic injury, which may prompt postoperative ileus, bowel perforation, and fistula [9]. However, no author has reported any complications that may have been caused by inadequate circulation [10].
10.4.3 Peritoneal Cavity Expander
A variation of the open HIPEC-described by Fujimura et al. and mainly used in Japan for treating or preventing gastric carcinomatosis—is the peritoneal cavity expander (PCE) technique (Fig. 10.3) [11]. The PCE is an acrylic cylinder containing inflow and outflow catheters that is secured over the wound. When filled with heated perfusate, the PCE can accommodate the small bowel, allowing the small intestine to float freely and be manually manipulated in the perfusate. The expander theoretically allows more uniform distribution compared with the closed technique. Its main disadvantage is the risk of OR personnel exposure to the chemotherapeutic agent, as occurs with the coliseum technique [10].
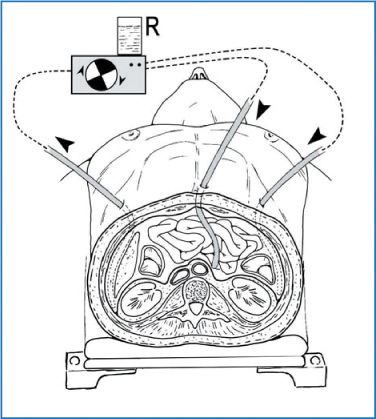

Fig. 10.3
Hyperthermic intraperitoneal chemotherapy (HIPEC): closed technique. (Courtesy of Prof. Angelo Di Giorgio)
10.4.4 Semiopen (or Semiclosed) Abdominal Technique
To create a watertight environment, edges of the incision are tightly stapled with a soft abdominal cavity expander supported by a Thompson self-retaining retractor positioned over the abdomen. This permits the level of the liquid to rise above the level of the skin edges. Edges of the anterior-wall peritoneum are constantly exposed to the liquid. Large-amplitude movements become possible: the surgeon can introduce both forearms, even both arms, into the patient’s abdomen without causing any liquid loss [12, 13].
10.5 Early Postoperative Intraperitoneal Chemotherapy
A second method of performing perioperative intraperitoneal chemotherapy is early postoperative intraperitoneal chemotherapy (EPIC). This approach is not favored by most surgical centers involved in treating carcinosis (Table 10.1). Chemotherapeutic agent administration is started immediately after the operation and continued during the first 1–5 postoperative days [14]. The EPIC system is composed of a Tenckhoff catheter or a subcutaneous port placed through the abdominal wall in the approximate area at greatest risk of recurrence following cytoreductive surgery. Closed suction drains are placed in dependant areas in the pelvis and below each hemidiaphragm. EPIC has the advantages of administering multiple chemotherapy cycles and increased exposure of tumor cells to therapy, as the chemotherapeutic drug is not drained for at least 24 h. However, there is greater opportunity for significant systemic absorption and its resultant adverse effects, as the chemotherapeutic agents remains in the peritoneal cavity for such a long period. Using drugs with a high first-pass effect after portal absorption—such as 5-fluorouracil (5-FU), the most common drug used with this technique-partially overcomes this problem [7]. Moreover, other complications related to long-term catheters (infections, bowel obstruction) are reported: EPIC significantly increased the rate of postoperative complications in the large, multicentric retrospective study of 504 patients with colorectal carcinomatosis treated with cytoreductive surgery combined with perioperative intraperitoneal chemotherapy [15].
Table 10.1
Different drug regimens applied in different peritoneal surface malignancy centers. (Modified from [26])
Indication | HIPEC: dose/perfusion time | Concomitant IV therapy | EPIC | Center |
|---|---|---|---|---|
Appendiceal and colorectal carcinomatosis | Mitomycin C, 15 mg/m2 doxorubicin, 15 mg/m2/90 min | 5-FU, 400 mg/m2; LV, 20 mg/m2 | 5-FU 4 days | Washington Hospital Center, Washington, DC (USA) |
Oxaliplatin, 130 mg/m2/60 min | 5-FU, 400 mg/m2; LV, 20 mg/m2 | 5-FU 4 days | Washington Hospital Center, Washington, DC (USA) | |
Oxaliplatin, 460 mg/m2/30 min | 5-FU, 400 mg/m2; LV, 20 mg/m2 | No | Gustave Roussy Institute, Villejuif (France) | |
Mitomycin C, 35 mg/m2/60 min | No | No | National Cancer Institute, Amsterdam (The Netherlands) | |
Mitomycin C, 3.3mg/m2/L cisplatin, 25 mg/m2/L/60 min | No | No | National Cancer Institute, Milan (Italy) | |
Mitomycin C,10 mg/mL perfusate/60 min | No | No | Centre hospitalo-universitaire | |
Gastric cancer | Cisplatin, 50 mg/m2 doxorubicin, 15 mg/m2/90 min | 5-FU, 400 mg/m2; LV, 20 mg/m2 | Taxol 4 days | Washington Hospital Center, Washington, DC (USA) |
Mitomycin C,10 mg/mL perfusate/60 min | No | No | Centre hospitalo-universitaire Lyon-sud, Lyon (France) | |
Peritoneal mesothelioma | Cisplatin, 50 mg/m2 doxorubicin, 15 mg/m2/90 min | 5-FU, 400 mg/m2; LV, 20 mg/m2 | Taxol 4 days | Washington Hospital Center, Washington, DC (USA) |
Cisplatin, 43 mg/L doxorubicin ,15.25 mg/mL/90 min | No | No | National Cancer Institute, Milan (Italy) | |
Mitomycin C, 0.5 mg/kg cisplatin 0.7 mg/kg/60 min | No | No | Centre hospitalo-universitaire Lyon-sud, Lyon (France) | |
Cisplatin, 250 mg/m2/90 min | No | 5-FU Taxol 1 days | National Cancer Institute, Bethesda, MD (USA) | |
Adverse ovarian cancer | Cisplatin, 50 mg/m2 doxorubicin, 15 mg/m2/90 min | 5-FU, 400 mg/m2; LV, 20 mg/m2 | Taxol 4 days | Washington Hospital Center, Washington, DC (USA) |
Cisplatin, 43 mg/L doxorubicin,15.25 mg/mL/90 min | No | No | National Cancer Institute, Milan (Italy) | |
Cisplatin, 20 mg/m2/L/90 min | No | No | Centre hospitalo-universitaire Lyon-sud, Lyon (France) |
EPIC efficacy is limited by adhesion formation, which can cause pooling of the chemotherapeutic agent in limited parts of the abdomen, with consequent systemic toxicity; also, this treatment is not performed with hyperthermia. In fact, heat is cytotoxic in vitro at 42.5°C [16], and hyperthermia enhances the antitumor effect of agents such as oxaliplatin, mitomycin, doxorubicin, and cisplatin by augmenting cytotoxicity and increasing drug penetration into tissue [17–19]. Elias et al. compared two similar groups of patients with colorectal peritoneal carcinomatosis, one treated with EPIC using 5-FU and mitomycin C, and one treated with HIPEC using oxaliplatin at 43.8°C (43°C). Mortality, morbidity, peritoneal recurrence, and overall survival rate all favored the HIPEC group. In particular, peritoneal recurrence was reported as being doubled in EPIC group compared with the HIPEC group [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree