Hyperparathyroidism
Hyperparathyroidism is a common disorder of calcium, phosphorus, and bone metabolism caused by increased circulating levels of parathyroid hormone (PTH). This disease is important to geriatricians because it occurs with increasing frequency in older patients. Widespread use of multiphasic biochemical screening tests led to an increase in the incidence of cases of primary hyperparathyroidism. In the population in Olmstead County, Minnesota, which is served by the Mayo Clinic, the annual incidence rose from 16 per 100 000 people before 1978 to a peak of 112 per 100 000 people 7 years later, then the rate declined. Now such screening tests are used less frequently and the incidence has fallen to 4 per 100 000 people. It is not clear why the incidence of hyperparathyroidism is decreasing, but it is postulated that there is a decreased exposure to ionizing radiation, or better supplementation with vitamin D.
Primary hyperparathyroidism may occur at any age but is found most commonly between the ages of 40 and 65 years. The incidence is approximately 1 per 1000. The disease affects women more than men by almost 2:1, and in women, this usually occurs in the first decade after menopause. In the United States, approximately 80% of patients with primary hyperparathyroidism have no signs or symptoms that are referable to their disease. With the development of sophisticated and specific technologies during the past 15 years, it has become feasible to evaluate patients with asymptomatic primary hyperparathyroidism in ways that have helped to establish prudent guidelines for surgical or medical management.
In hyperparathyroidism, PTH is inappropriately secreted by single or multiple glands in the presence of increased serum calcium levels. The disease is considered primary when autonomous hypersecretion of PTH is caused by a single adenoma, diffuse hyperplasia, multiple adenomas, or, rarely, a parathyroid carcinoma. Secondary hyperparathyroidism occurs when there is a prolonged hypocalcemic stimulus, as in cases of vitamin D deficiency or chronic renal failure. Tertiary hyperparathyroidism occurs in patients with chronic secondary hyperparathyroidism who develop autonomous hypersecretion of PTH and hypercalcemia, e.g., patients who undergo successful kidney transplants. This chapter focuses on primary hyperparathyroidism only.
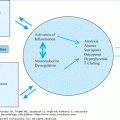
The etiology of primary hyperparathyroidism is unknown. When calcium is infused into hypercalcemic hyperparathyroid patients, there is a failure to suppress the PTH levels. Furthermore, when cells from hyperparathyroid glands are incubated in vitro, higher levels of ionized calcium in the medium are required to suppress PTH release than are required to suppress PTH release from cells from normal glands. These data suggest that, in part, the abnormality occurring in the parathyroid gland is an elevation of the set point at which ionized calcium levels suppress PTH release. The receptor that is responsible for calcium-sensing in parathyroid glands has been cloned; it is a guanosine triphosphate (GTP)-binding protein that has a seven-amino-acid transmembrane domain. Mutations in this receptor are rare in primary hyperparathyroidism.
In most cases of hyperparathyroidism, no etiologic agent can be identified; these represent sporadic cases. Previous neck exposure to ionizing radiation is associated with an increased incidence of hyperparathyroidism. Lithium, when used for therapy of bipolar disorders, is associated with hypercalcemia and increased PTH levels in up to 10% of patients. Thiazide diuretics can cause hypercalcemia, but the persistence of hypercalcemia 6 weeks after stopping a thiazide usually means the agent has unmasked primary hyperparathyroidism. A few causes of hyperparathyroidism, usually parathyroid hyperplasia, are familial disorders that have an autosomal dominant mode of transmission, such as (1) familial hyperparathyroidism, (2) multiple endocrine neoplasia type I (Werner’s syndrome: hyperparathyroidism, islet cell tumors, and pituitary tumors), and (3) multiple endocrine neoplasia type II (Sipple’s syndrome: medullary carcinoma of the thyroid, pheochromocytoma, and hyperparathyroidism). Only rarely does hyperparathyroidism occur in multiple endocrine neoplasia type IIB or III (medullary carcinoma of the thyroid, pheochromocytoma, mucosal neuromas, and marfanoid body habitus).
The pathologic abnormality in the parathyroid gland(s) maybe an adenoma, four-gland hyperplasia, multiple adenomas, or carcinoma. Single adenomas cause 80% of the cases of hyperparathyroidism. Hyperplasia of all four glands is found in 15% of cases; parathyroid carcinomas and multiple adenomas comprise the remainder. Determining whether a single gland is an adenoma or chief cell hyperplasia is often difficult to ascertain by histologic features alone, so the gross pathology seen at operation is necessary to classify the disease. An adenoma is diagnosed when only one abnormal gland is found (all other glands are normal). Chief cell hyperplasia is diagnosed when more than one abnormal gland is found. Controversy currently exists regarding whether it is possible to have multiple adenomas. In several studies, enlargement of only two glands was documented, with the remaining two being normal. A rarer form of parathyroid hyperplasia is called “water-clear cell” hyperplasia, in which large, membrane-lined vesicles fill the cytoplasm. Finally, parathyroid carcinoma is diagnosed by finding mitotic figures in the gland or finding capsular or vascular invasion in pathological specimens obtained during surgery.
Patients with primary hyperparathyroidism can present with a varying spectrum of signs and symptoms ranging from a total lack of symptoms to acute hypercalcemic crisis. The diagnosis is most frequently made by routine calcium measurements with multichannel screening chemistries in a patient with either no symptoms or only weakness or easy fatigability. Acute hypercalcemic crisis is now a rare form of presentation.
Osteitis fibrosa cystica, with radiographic features of osteopenia, subperiosteal bone resorption, brown tumors, bone cysts, and “salt and pepper” skull, is rare. Patients with hyperparathyroidism show evidence of increased bone remodeling on bone biopsy, with increased amounts of osteoid surface and eroded surface when compared to normal subjects. However, dynamic parameters of bone remodeling show that the mineral apposition rate is unchanged. Although radiographic evidence of hyperparathyroid bone disease in hand films—with subperiosteal resorption and loss of the distal tuft of the phalanges—is rare in cases of primary hyperparathyroidism now, it is found in patients with secondary hyperparathyroidism from chronic renal failure.
Because osteoporosis is such a major health problem in older patients, attention has been directed at determining PTH’s effect upon bone mass. With improved techniques to measure bone mass, it is possible to assess changes in both trabecular and cortical bone envelopes. Several early cross-sectional studies suggested that hyperparathyroid subjects have decreased amounts of trabecular bone in the vertebrae. More recent work shows that cortical bone as measured in the forearm or femur is reduced in the affected patients. Thus, elevated levels of PTH seem to have different effects upon cortical bone. Four studies suggest that patients with hyperparathyroidism have an increase in vertebral compression fractures, but one study found no evidence of an increased incidence of vertebral fractures. A study in Sweden of more than 1800 patients reported no increase in hip fractures in women with hyperparathyroidism, but there was an increase in hip fractures in men.
Nephrolithiasis occurs in 20% of hyperparathyroid patients. Of patients with kidney stones, approximately 5% have hyperparathyroidism. PTH causes a proximal renal tubular acidosis, increasing bicarbonate loss and decreasing hydrogen ion excretion, as well as lowering the phosphate reabsorption threshold. These changes cause a hyperchloremic metabolic acidosis, and 30% of patients will be hypophosphatemic. Hyperparathyroidism can cause nephrocalcinosis and a subsequent decline in the glomerular filtration rate. Hypercalcemia can lead to nephrogenic diabetes insipidus because the renal tubule becomes unresponsive to the action of antidiuretic hormone. Asymptomatic patients with primary hyperparathyroidism have defects in their ability to concentrate urine.
Most other signs and symptoms of hyperparathyroidism can be attributed to the resultant hypercalcemia or, more specifically, the elevated ionized calcium level. Gastrointestinal (GI) disorders include anorexia, nausea, vomiting, and constipation. Peptic ulcer disease occurs with increased frequency, and, rarely, it maybe the first clue to a multiple endocrine neoplasia type I syndrome (hyperparathyroidism, islet cell tumors, especially gastrinoma, and, finally, pituitary tumors). Pancreatitis can also occur or be exacerbated by the hypercalcemia. Central nervous system disorders include impaired cognition, recent memory loss, anosmia, depression, lethargy, and coma. Thus, hypercalcemia and hyperparathyroidism are rare but important considerations in the differential diagnosis of depression and dementia in the elderly. Neuromuscular disturbances include a proximal weakness, more prominent in lower than in upper extremities. Many patients complain of malaise and fatigue. Rarely, pruritus can be caused by metastatic calcification in the skin. Articular disturbances include pseudogout from calcium pyrophosphate crystal deposition in articular cartilage, calcific tendinitis, and chondrocalcinosis. The main cardiovascular disturbance is an increased frequency of hypertension. Stefenelli et al. report that patients with surgically confirmed primary hyperparathyroidism showed a high incidence of left ventricular hypertrophy (82%) and aortic and/or mitral valve calcifications (46% and 39%, respectively). At 41 months after successful parathyroidectomy, there was regression of the left ventricular hypertrophy. Further studies are needed to confirm and expand upon these observations.
Physical signs are unusual in hyperparathyroidism. Soft-tissue calcification can cause pseudogout or cutaneous calcification. When present in the eye, deposits of calcium phosphate crystals can cause conjunctivitis. In the cornea, band keratopathy (a vertical line of calcium phosphate deposition parallel to and within the ocular limbus) is best appreciated with a slit lamp examination. Enlarged parathyroid glands are difficult to palpate in the neck; generally, when a nodule is found in the neck of a suspected hyperparathyroid patient, it represents thyroid rather than parathyroid tissue.
Primary hyperparathyroidism is diagnosed by elevated serum calcium levels and, frequently, associated hypophosphatemia, without any other apparent disease or drug causing the abnormalities. The serum calcium should be measured fasting on several occasions with minimal or no venous stasis. Techniques for measuring PTH have improved significantly, making it possible to diagnose hyperparathyroidism directly rather than by exclusion as had been done previously. Thus, to prove hyperparathyroidism, serum PTH levels should be measured directly. Early assays measured the carboxy terminal portion of PTH. Because this fragment is cleared by the kidney, the diagnosis of hypercalcemia in patients with renal insufficiency was confounded. With improvement in assay techniques for the intact PTH molecule, using immunoradiometric (IRMA) and immunochemiluminescent (ICMA) assays, it is possible to show PTH elevations in 90% of patients.
Most clinical PTH assays have been validated so that the laboratory provides a range reflecting previous experience with the assay and showing where the patient’s PTH and serum calcium levels fall in relation to the laboratory’s other cases of hyperparathyroidism. In most nonparathyroid causes of hypercalcemia, PTH levels are suppressed, except in the unusual case of a malignant neoplasm that produces PTH. As is discussed in the section on “Differential Diagnosis,” malignancy-associated hypercalcemia is always a concern in the differential diagnosis of hypercalcemia, but neoplasms that are actually proved to produce active PTH are unusual, most such cases being renal, pancreatic, ovarian, or hepatic carcinomas. In such instances, the PTH-related peptide secreted by the tumor does not usually cross-react with PTH of parathyroid origin in the immunoassays employed in most laboratories.
Serum phosphate levels maybe low in hyperparathyroidism, but they can be normal, especially if there is renal impairment. Although PTH does cause phosphaturia, other factors such as dietary intake and time of day may affect renal phosphate handling. Furthermore, patients with malignancy-associated hypercalcemia can have a decrease in the renal phosphate reabsorption threshold from hypercalcemia per se or from the tumor-derived peptides, which produce the hypercalcemia. Other serum electrolyte abnormalities, such as elevated chloride, low bicarbonate, and low magnesium levels, are not specific enough to be of diagnostic value. Both an elevated serum alkaline phosphatase level and increased urinary hydroxyproline level suggest significant skeletal involvement from hyperparathyroidism.
Patients who are being evaluated for hyperparathyroidism should have a 24-hour urine calcium and creatinine excretion measured. Patients with a calcium:creatinine ratio of 0.1 may have familial hypercalcemic hypocalciuria, a hereditary disorder with normal PTH levels that does not require surgery. It is caused by an inactivating mutation of the calcium-sensing receptor.
Routine use of preoperative localization of abnormal parathyroid tissue in hyperparathyroidism should not be part of the diagnostic evaluation because noninvasive imaging techniques require further development before being valid for such application. Some centers are evaluating the use of technetium-99m sestamibi scans or ultrasonography, but they should not be used routinely in evaluating patients for parathyroidectomy. Arteriography and selective venous catheterization looking for “stepped-up” levels is a technically difficult procedure and should be performed by experienced hands only when hyperparathyroidism persists after a failed neck exploration.
The differential diagnosis of hyperparathyroidism is that of hypercalcemia, which can be caused by a diverse group of diseases and drugs (Table 111-1). A major concern when hypercalcemia is encountered is whether it is caused by a neoplasm. The clinical setting must be considered. Most patients with malignancy-associated hypercalcemia have obvious neoplastic disease on thorough examination and routine diagnostic workup. Thus, a chest x-ray a mammogram, and a serum and urine protein electrophoresis should be ordered when evaluating hypercalcemia. Since primary hyperparathyroidism is a common disease in older women, an elderly female with hypercalcemia without obvious evidence of malignant disease will be more likely to have primary hyperparathyroidism than occult malignancy.
Primary hyperparathyroidism |
Solitary adenoma |
Hyperplasia |
Multiple endocrine neoplasia |
Malignancy-associated hypercalcemia |
Local osteolytic hypercalcemia |
Humoral hypercalcemia of malignancy |
1,25(OH)2D-mediated hypercalcemia |
Granulomatous disorders |
Sarcoidosis |
Berylliosis |
Tuberculosis |
Histoplasmosis |
Coccidiomycosis |
Candidiasis |
Eosinophilic granuloma |
Silicone implants |
Medications |
Vitamin D and A intoxication |
Lithium |
Thiazide diuretics |
Estrogens/antiestrogens |
Theophylline |
Immobilization plus |
Juvenile skeleton |
Malignancy |
Paget’s disease of bone |
Primary hyperparathyroidism |
Renal failure |
Milk alkali syndrome |
Parenteral nutrition |
Familial hypocalciuric hypercalcemia |
Hypophosphatemia |
Renal failure |
Idiopathic hypercalcemia of infancy |
Hyperthyroidism |
Addison’s disease |
Hyperproteinemia |
Familial hypocalciuria hypercalcemia (FHH) should be considered in an evaluation of hypercalcemia. Although uncommon, FHH can present with hypercalcemia, but there is usually a family history, reflecting an autosomal dominant mode of inheritance, and 24-hour urinary calcium:creatinine excretion ratio of 0.1 is highly suggestive. At present, no adverse effects of the hypercalcemia have been reported from affected kindreds under supervision, and parathyroidectomy does not alter the hypercalcemia.
Drugs that cause hypercalcemia, such as thiazide diuretics and calcium supplements, can be excluded by withdrawing them for 4 weeks and making sure that serum calcium levels return to normal. Hypercalcemia caused by vitamin D intoxication can be diagnosed by measuring 25-hydroxyvitamin D levels and finding a level above 120 ng/mL. Hypercalcemia can be found in sarcoidosis, tuberculosis, and chronic fungal infections. The mechanism in all these diseases is believed to be increased production of 1,25-dihydroxyvitamin D by the granulomatous tissue, which causes increased calcium absorption from the GI tract. Other diseases causing hypercalcemia, such as hyperthyroidism, adrenal insufficiency, and vitamin D intoxication, should be diagnosed by their historical or clinical features.
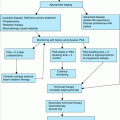
Treatment of hyperparathyroidism depends upon the way in which the patient presents to the physician. Because most cases are asymptomatic at presentation, no immediate therapy is usually necessary and a thorough diagnostic evaluation can be undertaken. When the patient presents with a hypercalcemic crisis (e.g., obtunded with serum calcium levels of greater than 12 mg/dL), management of the hypercalcemia must take precedence over diagnostic studies. Most hypercalcemic patients are dehydrated and may require several liters of parenteral fluids to lower the serum calcium into the 11.0 mg/dL range. Once hydration has been reestablished and the patient is stable, further decisions about therapy can be made.
At this time, there is no effective medical therapy for primary hyperparathyroidism. Beta-blockers, estrogen therapy in postmenopausal women, phosphate supplementation with potassium phosphate (Neutra-Phos-K), etidronate disodium (Didronel), or oral cellulose phosphate with dietary calcium restriction may lower serum calcium levels, while other aspects of the disease may progress. The second-generation bisphosphonates, alendronate, and risedronate can lower serum calcium levels, but long-term studies are needed to document their effect on the disease and whether they alter fracture rates. A new class of drug, calcimimetic agents, is under study. Calcimimetics activate the calcium sensing receptor in the parathyroid gland and inhibit PTH secretion. A long active calcimimetic agent, cinacolcet, is now approved by the Food and Drug Administration (FDA) for the treatment of secondary hyperparathyroidism with renal failure and for hypercalcemia from parathyroid cancer. This agent is not approved for the treatment of primary hyperparathyroidism, but early results suggest it may help control serum calcium levels. Therefore, long-term management of hyperparathyroidism must involve a decision about whether to intervene surgically or to follow the patient until there is an indication for surgery.
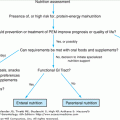
Because many cases of hyperparathyroidism are asymptomatic and without any potential complications of the disease at diagnosis, immediate surgery is not necessary, and some patients may never need an operation. A National Institutes of Health (NIH) consensus conference points out that because surgery is the only effective therapy for this disorder, the patient and the physician must realize that meticulous, long-term follow-up is necessary. Understanding of long-term complications is incomplete, and no study has randomized asymptomatic patients to surgery or medical follow-up.
There are indications for surgery in asymptomatic patients with primary hyperparathyroidism: (1) markedly elevated serum calcium (above 12.0 mg/dL); (2) history of life-threatening hypercalcemia; (3) reduced creatinine clearance (below 30% for age-matched normals); (4) nephrolithiasis; (5) markedly elevated 24-hour urinary calcium excretion (above 400 mg); and (6) reduced bone mass as measured by direct measurement (more than 2 standard deviations below age-matched normals). Surgery should be considered strongly in the following circumstances: (1) the patient desires surgery; (2) meticulous, long-term follow-ups are unlikely; (3) coexistent illness complicates management; and (4) the patient is young (younger than age 50 years). After successful surgery, recovery is rapid. Serum calcium levels normalize within hours to several days. There is a 90% reduction in recurrent renal stones in patients with nephrolithiasis. Bone mass also improves after surgery; patient can gain 12% to 20% of their femoral or lumbar density respectively in 4 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree