Genus/Species
AmB
Itraconazole
Voriconazole
Posaconazole
Anidulafungin
Caspofungin
Micafungin
Ref.
Fusarium spp.
F. oxysporum
F. solani
++
++
+
+
+
+
++b
++++
+
++
++
+
0
ND
ND
0
ND
ND
ND
ND
ND
Scedosporium spp.
S. prolificans
S. apiospermum
0
0
0
++
0
++++
0
+++
0
0
ND
0
ND
ND
[60]
Paecilomyces spp.
++
+++
+++
+++
++
+++
ND
Acremonium spp.
++
0
++
ND
0
ND
ND
[60]
Scopulariopsis spp.
0
0
0
0
+
+
+++
[55]
Trichoderma spp.
++
0
+
0
+++
+++
+++
[58]
Fusarium Species
Fusarium species have emerged as a common cause of disseminated fungal infections in neutropenic patients and those undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Fusarium represents the second most common fungal pathogen, after Aspergillus, as the cause of life threatening infection in recipients of hematopoietic transplant [2]. Because of this, Fusarium infections are sometimes discussed separately from hyalohyphomycosis as fusariosis. Fusarium causes a broad spectrum of infections in humans, including superficial and local infections in immunocompetent hosts; disseminated infections are seen almost exclusively in immunosuppressed patients.
Etiologic Agents
Four species are most commonly involved in human infections: F. solani (most common), F. oxysporum, F. verticillioides (moniliforme), and F. proliferatum [3]. F. solani species complex also includes F. falciforme (formerly Acremonium falciforme) and F. lichenicola (formerly Cylindrocarpon lichenicola). Fusarium species are septate filamentous fungi that produce conidiophores, phialides, macroconidia, and microconidia. Fusarium species are readily and rapidly recovered in almost all fungal media. On potato dextrose agar (PDA), the colonies have a velvety or cottony surface, and are white, yellow, pink, purple salmon, or gray on the surface, with a pale red, violet, brown, or sometimes blue reverse. The characteristic sickle- or banana-shaped multiseptate macroconidia with a foot cell at the base are used in identifying the genus and species of Fusarium (Fig. 11.1). Molecular methods may also be used for rapid identification of Fusarium to the species level. In tissue, the hyphae are similar to those of Aspergillus species, with hyaline and septate filaments that typically dichotomize in acute and right angles. In the absence of microbial growth, distinguishing fusariosis from aspergillosis and other hyalohyphomycoses is difficult, and requires the use of in situ hybridization in paraffin-embedded tissue specimens [4]. Fusarium species are toxigenic, and may cause mycotoxicosis in animals and humans [3].
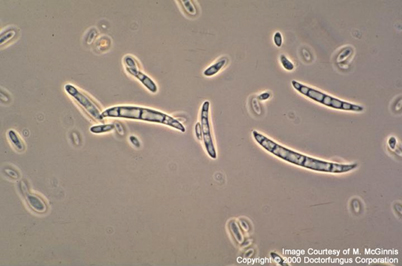
Fig. 11.1
Fusarium oxysporum macroconidia. Characteristic fusiform to sickle-shaped, multiseptate mostly with an attenuated apical cell and a foot-shaped basal cell. (Courtesy of www.doctorfungus.org © 2000)
Epidemiology
Fusarium is ubiquitous in soil and water, taking part in water biofilms, and is a human and plant pathogen [5]. Fusarium species are causative agents of superficial and localized infections in immunocompetent hosts, most commonly onychomycosis and cutaneous and subcutaneous infections, including mycetoma and keratitis, the latter in contact lens wearers [6]. A recent large outbreak of Fusarium keratitis was reported in contact lens wearers in the USA and was linked to contaminated contact lens rinse solutions. Other risk factors for keratitis are trauma and use of topical corticosteroids and antibiotics [7]. Fusarium endophthalmitis may arise from keratitis or by direct inoculation after cataract surgery or trauma [8]. Fusariosis may also result from skin breakdown, such as burns and wounds, or the presence of foreign bodies, such as peritonitis in patients receiving continuous ambulatory peritoneal dialysis (CAPD), catheter-associated fungemia, and thrombophlebitis [9–11]. A hospital outbreak of F. verticillioides fungemia in immunocompetent patients has been recently reported [12]. Other infections include sinusitis, pneumonia, cutaneous and subcutaneous infections, septic arthritis, and osteomyelitis [13–17].
Immunosuppressed patients may develop locally invasive and disseminated fusariosis [18]. Risk factors include prolonged neutropenia, such as following chemotherapy for acute leukemia, and T cell immunodeficiency, which occurs most commonly after HSCT [18–20]. In HSCT, infection may develop early during neutropenia or months after neutrophil recovery following the treatment of chronic extensive graft versus host disease (GvHD). Localized infections may also develop among solid organ transplant recipients (SOT), usually as a late infection [21].
Portals of entry are the respiratory tract and skin, the latter playing a significant role in patients with tissue breakdown such as onychomycosis. Hospital water systems are a potential reservoir for Fusarium; transmission may occur from inhalation of conidia aerosolized in the shower or from direct contact of contaminated water with sites of skin breakdown [22–24].
Pathogenesis and Immunology
Similar to Aspergillus, this organism is highly angioinvasive and leads to tissue infarction. In contrast to Aspergillus, however, Fusarium is frequently isolated from the bloodstream, likely as a result of intravascular adventitious sporulation [25]. Phagocytes appear to be the predominant line of defense against fusarial infections [18–20].
Clinical Manifestations
Infection with Fusarium in immunocompetent hosts may be superficial or locally invasive, involving the skin, eyes, sinuses, lungs, and joints and bones. In immunosuppressed patients, the infection may be locally invasive, usually pneumonia and/or sinusitis, or more commonly disseminated [18–20]. The clinical picture resembles that of invasive aspergillosis. Unlike aspergillosis, however, fungemia and skin lesions are common (up to 40 % of patients with disseminated disease). Skin lesions may represent the primary site of infection (onychomycosis) or secondary to disseminated infection [18–20]. Recovery of Fusarium from preexisting skin or nail lesions has been reported as a risk factor for invasive disease in high-risk patients [26]. Metastatic skin lesions evolve from subcutaneous painful lesions to erythematous induration followed by ecthyma gangrenosum-like necrotic center, which may be surrounded by a rim of erythema [18–20].
Diagnosis
Two characteristics suggest the diagnosis of disseminated fusariosis in the severely immunocompromised host: metastatic skin lesions and positive blood cultures for mould [18–20]. Definitive diagnosis relies on cultures (tissue and/or blood) and histopathology which show a pattern common to all hyalohyphomycosis (invasion by acute-branching, septate hyaline hyphae). The use of PCR techniques and/or in situ hybridization may be required to reach the correct diagnosis in tissues [4, 27]. The 1,3-β-d-glucan test is usually positive in invasive fusarial infections, but it cannot distinguish Fusarium from other fungal infections (Candida, Aspergillus, and others) which are also detected by the assay [28].
Treatment
Localized infections, particularly in immunocompetent hosts, usually respond well to treatment consisting of topical therapy for fungal keratitis or excision of involved tissue (sinuses, eye, soft tissue, bone). Removal of an infected intravascular catheter may be needed in the rare cases of catheter-related fungemia.
Outcome of invasive and disseminated fusariosis in immunosuppressed patients remains quite poor, but appears to have improved with the introduction of voriconazole and posaconazole [18–20, 29, 30]. Predictors of poor outcome are persistent neutropenia and recent therapy with corticosteroids for chronic GvHD [19]. Treatment options are limited by the lack of reliable and consistent activity of antifungal agents against Fusarium species. Susceptibility varies among the various species, with resistance seen to all three major classes of antifungal agents [31]. Rapid species identification may be helpful, but antifungal susceptibility testing should be considered because of this variable in vitro susceptibility among Fusarium species (Table 11.1). The echinocandins do not appear to be active against any Fusarium species
Pseudallescheria and Scedosporium Species
Previously, only two Scedosporium species, S. apiospermum (sexual state name, Pseudallescheria boydii) and S. prolificans (formerly S. inflatum), were described as human fungal pathogens [32, 33]. The fungi previously denoted P. boydii (asexual state name, S. apiospermum) have now been divided into at least four species—P. boydii (S. boydii), P. minutispora, S. aurantiacum, and S. dehoogii [34]. Some have called these newly described (renamed) fungi the P. boydii complex. In this text, we refer to this group as P. boydii. A spectrum of disease, ranging from respiratory tract colonization to superficial and deep infections, in both immunocompetent and immunosuppressed hosts, has been reported. Rarely, disseminated infection with high mortality is seen in the setting of severe immunosuppression. S. prolificans belongs to the group of fungi which causes phaeohyphomycosis (dematiaceous fungi), but will be briefly discussed because of its relation to P. boydii.
Etiologic Agents
Scedosporium species are identified by their characteristic macroscopic (wooly to cottony, dark gray to dark brown) and microscopic (characteristic conidia, conidiophores, and hyphae) appearance. S. prolificans is distinguished from P. boydii by the production of terminal annelloconidia with inflated bases (cylindrical in S. apiospermum) and growth inhibition by cycloheximide or actidione. In tissue sections, Pseudallescheria/Scedosporium appear as septate hyaline hyphae that cannot be reliably distinguished from Aspergillus or Fusarium unless conidia are present.
Epidemiology
Pseudallescheria/Scedosporium have been isolated from soil, potting mix, compost, animal manure, and stagnant or polluted water. Infections occur worldwide, though a large number of reports come from Northern Spain [32]. Patients at risk for invasive and/or disseminated infection include those with HIV infection, acute leukemia, and recipients of allogeneic HSCT or SOT [35, 36].
Infection is thought to be secondary to direct inoculation (such as after trauma) or inhalation of airborne conidia. In normal hosts, P. boydii causes infection after penetrating trauma, including keratitis, endophthalmitis, cutaneous and subcutaneous infections, bursitis, arthritis, and osteomyelitis. Following near-drowning accidents, sinusitis, pneumonia, meningoencephalitis, and brain abscesses may develop. Allergic bronchopulmonary disease due to P. boydii may also occur. Like P. boydii, S. prolificans causes localized infections (usually of bone or soft tissue) in immunocompetent patients following trauma, and deeply invasive infections in immunocompromised hosts, sometimes as a nosocomial outbreak [32, 35, 36].
Clinical Manifestations
Mycetoma is the most common P. boydii infection in normal hosts (see Chap. 22), usually occurring after penetrating injury and presenting as lower extremity swelling with draining sinuses. Other infections include non-mycetoma cutaneous and subcutaneous infections, keratitis, and endophthalmitis [36]. Invasive P. boydii infection is usually seen in immunocompromised patients, most commonly as pneumonia. Disseminated infection is mainly associated with S. prolificans, and is characterized by refractory fever, pulmonary infiltrates (diffuse or nodular), central nervous system involvement (present in one third of patients), fungemia, renal failure, erythematous, and nodular skin lesions with central necrosis.
Diagnosis
The diagnosis relies on the combination of clinical signs and symptoms and recovery of Pseudallescheria/Scedosporium from blood and/or infected tissue, with or without demonstration of colorless septate hyphae.
Treatment
Localized infections, particularly in immunocompetent hosts, usually respond well to surgical debridement. Pseudallescheria boydii is resistant to fluconazole and flucytosine, but susceptible to the newer azoles—voriconazole, posaconazole, and ravuconazole (Table 11.1). Voriconazole is approved for use in P. boydii infections [37, 38]. Caspofungin appears to be more active than itraconazole or amphotericin B. Variable strain-to-strain susceptibility to amphotericin B can be seen [39]. Surgical resection remains the only definite therapy for S. prolificans infections, as this organism is resistant to all available antifungal agents in vitro. In vitro synergism between terbinafine and either voriconazole or itraconazole has been reported [40].
Paecilomyces and Purpureocillium Species
Paecilomyces and Purpureocillium species are frequent airborne contaminants in clinical microbiology laboratories but have been increasingly reported as cause of human infection.
Etiologic Agents
Two species, Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Paecilomyces varioti, account for most human infections. Paecilomyces marquandii and P. javanicus have also been reported to cause human disease. These fungi grow rapidly on various agar media including blood, chocolate, Sabouraud dextrose (SDA), and PDA. P. varioti is thermophilic and grows well at temperatures as high as 50 °C. The color of the colony and certain microscopic features help differentiate these fungal species from each other. The colonies are flat and velvety. The color is initially white and becomes yellow-green, yellow-brown, pink, or violet according to species.
Epidemiology
Paecilomyces and Purpureocillium are found worldwide in soil, food products, and water and causes infection in both immunocompetent and immunosuppressed patients. A strong association of these fungi with prosthetic implants may be due to their inherent resistance to most sterilizing techniques. Prosthetic implant-related infections include keratitis in contact lens wearers and after corneal implants (rarely endophthalmitis), and in recipients of CAPD, cardiac valves, and ventriculoperitoneal shunts. Other infections involve the nails, skin, subcutaneous tissues, bones and joints, sinuses, and lungs, while disseminated infections only occur in immunosuppressed patients [41].
Clinical Manifestations
The most common infections due to Paecilomyces and Purpureocillium involve the eye and eye structures (keratitis and endophthalmitis), followed by the nails (onychomycosis) and skin and soft tissues. Skin infections are characterized by erythematous macules, nodules, pustules, vesicular lesions, or necrotic crusts [41]. Sporotrichosis-like skin infection has also been described. Other reported infections in the competent host include peritonitis in CAPD, prosthetic-valve endocarditis, catheter-related fungemia, and arthritis/osteomyelitis. In immunocompromised patients, pneumonia and disseminated disease are most commonly observed.
Diagnosis
These fungi grow readily on routine fungal media, including SDA. Like all hyalohyphomycetes, they produce hyaline septate hyphae in tissue and can be seen with periodic acid–Schiff (PAS) staining in histopathology. Both may exist in various forms in tissue (conidia and phialides) and can therefore be misdiagnosed as candidiasis.
Treatment
Treatment of invasive Paecilomyces and Purpureocillium infections relies on surgical debridement and removal of infected prosthetic materials. Because of different susceptibilities to antifungal agents, these fungi should be identified to the species level (Table 11.1). P. varioti is susceptible to amphotericin B, flucytosine, itraconazole, voriconazole, and posaconazole, whereas P. lilacinum is only susceptible to the latter two triazoles [42].
Acremonium (and Gliomastix and Sarocladium) Species
Acremonium species are filamentous fungi of low pathogenicity commonly isolated from the environment (soil, insects, sewage, plants, and water) [43].
Etiologic Agents
Many species of Acremonium have been reported to cause human infection. Several of these have been transferred to the genera Gliomastix and Sarocladium. A. strictum is the most common of these species to cause of human infection, but infection has been attributed to A. alabamensis, A. potronii, A. recifei, G. roseogrisea, S. kiliense, and S. strictum [1]. One of the other more common species, A. falciforme, has been reclassified Fusarium falciforme. Acremonium species grow on SDA, forming white, salmon, or yellowish-green colonies that are usually velvety or cottony with slightly raised centers. This genus is distinguished by formation of narrow hyphae bearing solitary, unbranched needle-shaped phialides. Like other hyaline moulds, septate colorless hyphae are found in tissue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree