Chronic myeloid leukemia (CML) is the first cancer in which a genetic alteration was proven to be of pathogenic significance and is considered a disease model for oncogene addiction, targeted therapy, and cancer stem cells (CSCs). The introduction of tyrosine kinase inhibitors (TKIs) resulted in dramatic improvement in response and survival for patients with CML in chronic phase (CP); however, CSCs are spared by TKIs. In this article, we review the role of CSCs in CML in CP, their persistence following TKI treatment, and current approaches to target this population in an attempt to achieve disease cure.
Chronic myeloid leukemia (CML) is recognized as a disease model for oncogene addiction, targeted therapy, and cancer stem cells (CSCs). As the first cancer in which a genetic alteration was proven to be of pathogenic significance, CML research has remained at the leading edge, providing great insights into cellular and molecular biology and most recently in systems biology. CML, driven by genomic instability induced by the BCR-ABL oncogene, naturally progresses over time from chronic phase to accelerated phase (CP and AP) and eventually to blast crisis (BC). Following many frustrating years in the 1970s and 1980s, during which we used toxic strategies, such as high-dose chemotherapy, allogeneic stem cell transplantation (alloSCT) and autologous transplantation, interferon-α (IFN-α), and cytosine arabinoside, the late 1990s saw the introduction of targeted therapies, the tyrosine kinase inhibitors (TKIs), including imatinib mesylate (IM) (Gleevec, Novartis, Basel, Switzerland), dasatinib (DAS) (Sprycel, Bristol-Myers Squibb, New York, NY, USA), and nilotinib (NIL) (Tasigna, Novartis, Basel, Switzerland). These agents have dramatically altered the natural history of CML in CP, such that many individuals now experience a near normal quality of life with very significantly improved overall survival (OS). These agents are expensive, however, and therefore are not accessible to patients in many parts of the world. For patients fortunate enough to receive continuous treatment with TKIs, 30% to 60% experience grade 1–2 and 10% to 20% experience grade 3–4 side effects. Drug adherence studies also demonstrate reduced compliance over time, such that by 5 years of continuous therapy only 70% of patients are compliant and only 1 in 7 patients takes their medication exactly as instructed. In this situation, levels of compliance are very strongly correlated with disease response, suggesting that this is a critical issue for the ever-growing population of patients living with CML.
CML arises in a hematopoietic stem cell (HSC) as a result of the 9;22 translocation. The resultant BCR-ABL tyrosine kinase drives myeloid expansion through to terminally differentiated cells, explaining the disease phenotype. The disease follows a near normal differentiation hierarchy, with mutant HSCs (often referred to as leukemic stem cells [LSCs]) sustaining and driving the disease. This hierarchy is beautifully illustrated in patients on introduction of TKI therapy. In terms of leukemic burden, sensitively quantified by quantitative real-time polymerase chain reaction (Q-RT-PCR), there is a rapid decline explained by eradication of more mature BCR-ABL–positive cells, followed by a much slower reduction in CML progenitor cells to 3 years. Beyond 3 years, it is unclear whether the slope continues very slowly downward with cure predicted by 17 to 20 years, or whether disease levels plateau, presumably because CML LSCs represent a highly drug-refractory population. What is very clear is that CML, even in CP, is a highly heterogeneous disease and this may well explain the variable disease kinetics observed in individual patients treated with TKIs and in the small proportion of patients with CML who have discontinued TKIs after several years of therapy.
In this article, we aim to review the relevant literature and define the role of CML LSCs as a hurdle to cure of this disease.
Historical perspective of genesis and heterogeneity of cancer
Cancer is the result of perturbed cell growth processes. All tumors, whether solid or hematological, demonstrate substantial heterogeneity with respect to cell surface markers, morphology, genetic or epigenetic aberrations, cell growth kinetics, tumor-initiating capacity, and response to therapeutic agents.
Past perspectives defining cancer and how it originates have laid the groundwork for contemporary models addressing tumor heterogeneity. In the early 19th century, Johannes Muller and Rudolf Virchow hypothesized that cancer arises from embryo-like cells, thereby initiating early concepts of CSC. In 1963, stem cell pioneers Becker and colleagues described techniques involving a small population of normal hematopoietic cells that could form colonies in the spleen when injected into irradiated mice. They determined that these cells had the ability to self-renew and differentiate into mature hematopoietic cells, thus establishing basic stem cell properties. In 1976, Peter Nowel hypothesized that cancer may change its capacity to grow and metastasize as the result of accumulated genetic mutations that produce a dominant tumor clone, through diversification and natural selection processes, fundamentally modeling carcinogenesis on Darwinian principles. His landmark article suggested that tumor progression is based on neoplastic populations possessing an increased proliferative capacity and therefore exhibiting increased levels of fitness, leading to their selection. The universal acceptance that cancer is defined by abnormal growth control, resulted in therapies focused on augmented proliferation rates. Likewise, dosing and drug schedules were designed aiming for maximal tumor cell death with minimal toxicity to normal tissue.
Recently, a paradigm shift has occurred in the way cancer is perceived and how therapy might be redirected. This change in philosophy followed fundamental studies by Dick and colleagues demonstrating that acute myeloid leukemia (AML) contains a specialized population of cells expressing stem cell markers and exhibiting properties that can be propagated when transferred to immunocompromised NOD/SCID mice to cause leukemia. This was the first formal demonstration that cancer cells within a tumor may follow a normal stem cell hierarchy.
Contemporary models to account for tumor heterogeneity
Currently, 2 models that attempt to explain tumor heterogeneity dominate the field. The CSC model proposes that tumors follow a normal stem cell hierarchy, with CSCs having the sole ability to propagate themselves and reconstitute the entire tumor. In this model, CSCs are biologically distinct from the bulk tumor cells, can give rise to other CSCs through self-renewal, and can generate differentiated tumor cells. Proponents of this model emphasize that CSCs function as a distinct population, irrespective of their absolute frequency, with the primary tenets of the model being that the CSCs can be isolated, self-propagate, and initiate malignant growth. More importantly, findings suggest that the presence of CSCs drives clinical cancer progression and contributes to treatment failure. It is important to emphasize that a CSC need not originate from a normal tissue stem cell that has undergone transformation, but may instead arise in a more mature cell that has re-acquired gene expression and functional characteristics that support self-renewal. CSCs may arise from a stem cell, a committed progenitor, or even a terminally differentiated cell. Cancers supporting the CSC model include leukemia; breast, ovarian, bladder, central nervous system, colon, head and neck, pancreatic, and liver cancer; and Ewing sarcoma.
The clonal or stochastic model suggests that individual tumor cells are biologically equivalent, but subject to either intrinsic (transcription factors, regulatory pathways) or extrinsic factors (tumor milieu, immune response, microenvironment) that influence their fate. These indiscriminately induced, nonpermanent alterations to the cells eventually generate heterogeneity. In theory, every cell in the tumor is capable of propagating that tumor. Cancers that may follow this model include B-cell acute lymphoblastic leukemia and malignant melanoma. Most recently, advocates of both models concur in the literature that these cancer concepts are not mutually exclusive.
Contemporary models to account for tumor heterogeneity
Currently, 2 models that attempt to explain tumor heterogeneity dominate the field. The CSC model proposes that tumors follow a normal stem cell hierarchy, with CSCs having the sole ability to propagate themselves and reconstitute the entire tumor. In this model, CSCs are biologically distinct from the bulk tumor cells, can give rise to other CSCs through self-renewal, and can generate differentiated tumor cells. Proponents of this model emphasize that CSCs function as a distinct population, irrespective of their absolute frequency, with the primary tenets of the model being that the CSCs can be isolated, self-propagate, and initiate malignant growth. More importantly, findings suggest that the presence of CSCs drives clinical cancer progression and contributes to treatment failure. It is important to emphasize that a CSC need not originate from a normal tissue stem cell that has undergone transformation, but may instead arise in a more mature cell that has re-acquired gene expression and functional characteristics that support self-renewal. CSCs may arise from a stem cell, a committed progenitor, or even a terminally differentiated cell. Cancers supporting the CSC model include leukemia; breast, ovarian, bladder, central nervous system, colon, head and neck, pancreatic, and liver cancer; and Ewing sarcoma.
The clonal or stochastic model suggests that individual tumor cells are biologically equivalent, but subject to either intrinsic (transcription factors, regulatory pathways) or extrinsic factors (tumor milieu, immune response, microenvironment) that influence their fate. These indiscriminately induced, nonpermanent alterations to the cells eventually generate heterogeneity. In theory, every cell in the tumor is capable of propagating that tumor. Cancers that may follow this model include B-cell acute lymphoblastic leukemia and malignant melanoma. Most recently, advocates of both models concur in the literature that these cancer concepts are not mutually exclusive.
Techniques used to study CSCs
The ability to label cells according to specific cell surface markers using monoclonal antibodies combined with the capability to segregate specific populations based on their surface biomarker profile using fluorescence-activated cell sorting, significantly enhanced the study of purified CSC populations. Tumor-initiating cell assays performed on sorted cancer cell subpopulations do have their limitations; the act of segregating CSCs from the tumor bulk may have profound effects on cell-cell interactions and conversion of marker phenotype among CSC subpopulations (eg, CD133 has also been documented).
Serial xenotransplantation in animal models is regarded as the gold standard to determine self-renewal capacity as well as tumor re-establishment for human tumor-initiating cells. Unfortunately, human-to-mouse xenotransplantation has its own limitations, including residual components of the murine immune system, lack of cross-species reactivity of cytokines, and variations in the murine versus human microenvironment. These variables can have very significant ramifications on experimental results with regard to estimations of CSC frequency. For example, Quintana and colleagues demonstrated that by using more immunodeficient mice, namely the NOD/SCID interleukin-2 receptor gamma chain null strain that lack natural killer cell activity, tumourigenic melanoma cells may comprise almost 25% of the bulk population instead of the 1 in 1 million, as previously shown. This study in particular highlights limitations of xenotransplantation techniques with respect to estimating tumorigenic cell frequency and for extrapolating to the clinic for subsequent strategies for treatment.
Hurdles toward a cure for CML: the CML stem cell
Successful cancer therapies must target the cancer cells that are responsible for disease maintenance and progression. According to traditional cancer therapies and the clonal evolution hypothesis, the entire tumor bulk is targeted. In CML in CP, the introduction of TKIs, such as IM and second-generation agents, DAS and NIL, which induce cytogenetic and molecular responses in most patients, has dramatically altered the natural history of the disease. These agents are very effective in tumor debulking, and most patients with CML in CP achieve complete cytogenetic response (CCyR higher than 80% at 5 years), with progression-free survival and OS rates of more than 90%. Only 10% of CML CP cases achieve sustained complete molecular response (CMR) by 5 years. Therefore confirming that, although effective, IM fails to achieve full disease eradication.
Following the original demonstration of the presence of a population of primitive, quiescent LSCs in all patients with CML in CP, which is thought to reside in the CD34+38–Lin– population, there is a solid foundation of evidence in support of CML following a CSC model. We and others have demonstrated that CML stem and progenitor cells (eg, CD34+38–) are insensitive to induction of apoptosis by TKIs, although these agents exhibit potent antiproliferative effects. This observation is paralleled by clinical observations that BCR-ABL–positive cells are still detectable by Q-RT-PCR within the primitive HSC compartment (CD34+38–) of patients with CML, despite continuous and successful IM treatment ; moreover, when TKIs are interrupted in patients in CMR, more than 50% of patients will show evidence of molecular relapse. Mathematical models of the kinetics of the molecular response to IM also suggest that this drug inhibits production of differentiated leukemic cells, but does not deplete LSCs, although this conclusion has been challenged in other models and longer follow-up of patients on IM will be necessary to determine which model better fits the reality.
The CSC hypothesis proposes that targeting the elite subpopulation of CSCs responsible for recapitulating the tumor will yield successful therapy. Current evidence strongly suggests that the reason behind the inability of TKIs to cure CML in CP lies in the persistence of an LSC population whose survival is most likely dependent on unknown survival mechanisms inherent to the CML LSC. The real goal or absolute cure for CML should therefore involve drugs that will eliminate LSCs.
LSC resistance mechanisms
Oncogene addiction describes the dependency of tumor cells on a particular oncogene activity for growth and survival despite the presence of multiple genetic and epigenetic abnormalities. This phenomenon provides the rationale to develop targeted therapies. In CML, BCR-ABL has been considered to play such a crucial role and this has led to development of TKI. Following the original observation of the persistence of LSC following TKI treatment, it was initially hypothesized that this could be explained by the persistence of BCR-ABL kinase activity in this subpopulation of cells; however, evidence is now growing to suggest that BCR-ABL kinase-dependent resistance mechanisms might not be sufficient to fully explain this phenomenon.
Mutations in the BCR-ABL kinase domain have been described in patients with CML in CP, but account for only 10% to 20% of cases that show suboptimal or failed response to IM (fully reviewed in article 11). Furthermore, we have been unable to detect kinase domain mutations in CML LSCs that persist following prolonged TKI exposure in vitro (Hamilton and colleagues, unpublished data, 2011).
BCR-ABL transcripts are expressed at higher levels in LSCs as compared with more mature CML cells ; however, enhanced BCR-ABL inhibition with more potent TKIs is still unable to target quiescent LSCs, which remain viable and retain their clonogenic potential. These observations are paralleled by clinical trial findings from front-line treatment of patients with CML in CP with more potent TKIs. Although DAS and NIL produce higher rates of CCyR and major molecular response at early time points, CMR rates are still low at about 10%, and extended follow-up is required to establish whether these agents eradicate CML stem cells. This is despite full inhibition of BCR-ABL kinase activity in most patients.
Following reports that intracellular levels of IM in LSCs could be affected by either overexpression of the multidrug efflux transporter of the ATP-binding cassette (ABC) transporter family, MDR1, or reduced expression of the organic cation transporter hOCT-1, the possibility that BCR-ABL kinase activity was not effectively inhibited in LSCs as the result of reduced intracellular drug accumulation has also been investigated. For the efflux transporter ABCG2, a synthesis of experimental work suggests that although IM may be a substrate for ABCG2 at very low concentrations, at the concentrations that are relevant in the clinic, this drug acts as a potent ABCG2 inhibitor and would not therefore be effluxed from the cell. Most recently, we have shown that CML CP CD34+ cells barely express MDR1 and inhibiting MDR1 does not result in increased efficacy of IM in vitro. For hOCT-1, clinical observations suggest that high hOCT-1 activity is predictive of better response to IM. Once again, at the stem cell level this transporter is barely expressed ; moreover, NIL concentration in CML cells, including LSCs, does not seem to be affected by drug transporters. Therefore, the analysis of drug transporter expression and function on mature cells in patients with CML in CP before treatment might help in risk stratification and choice of TKI; however, it is unlikely to predict response at the stem cell level.
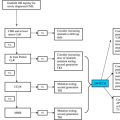
Although the presence of BCR-ABL kinase activity is necessary for leukemogenesis in vivo, the activated ABL kinase is not sufficient on its own to reproduce the full disease spectrum in mice, with multiple domains of BCR-ABL required to reproduce a CML-like disease. In contrast to other oncogenes, BCR-ABL fails to confer self-renewal and stem cell potential to committed murine hematopoietic progenitor cells. Recent collaborative research by several groups, including ours, has used a mouse model closely resembling human CML disease to show conclusively that leukemic long-term HSCs (LT-HSCs) are able to transplant disease, but that the BCR-ABL oncogene actually induces differentiation of the LT-HSCs and reduces their self-renewal potential. Leukemic LT-HSCs are preserved even when BCR-ABL expression is abrogated and can regenerate a CML-like disease on re-induction of BCR-ABL expression. Finally, we have now shown that DAS, a multitargeted TKI that potently inhibits both BCR-ABL and SRC, rapidly (within 1–4 hours) initiates the process of irreversible apoptosis in 80% to 90% of bulk CD34+ CML CP cells; however, the remaining 10% to 20% of CML CP stem cells (CD34+38– and long-term culture-initiating cells) remain insensitive to extended drug exposure (to 12 days), to increased drug concentration (from 10–1000 nM), or to withdrawal of supplemental cytokines from the serum-free medium (Hamilton and colleagues, unpublished data, 2011). Taken together, these data argue strongly that inhibition of BCR-ABL kinase activity alone is insufficient to eradicate CML LSCs and suggest that BCR-ABL, or at least its kinase activity, might provide a proliferative advantage to CML LSCs, but does not represent the main molecular mechanism for their maintenance, which may instead be embedded within the key survival pathways that are inherent to normal stem cells. As a consequence, one of the approaches currently being pursued to eradicate CML LSCs is to interfere with those pathways necessary for their maintenance ( Fig. 1 ).
The concept of oncogene addiction as a passive dependence of tumor cells on particular oncogene activity has also been challenged by other investigators who have proposed an alternative model to explain cancer cell death on inactivation of a particular oncogene. This model, called ‘oncogenic shock’ states that, on inhibition of a particular oncogene, disruption of several signaling pathways happens, which preferentially leads to apoptosis as prosurvival signals are abrogated before proapoptotic signals. The same investigators have shown that in a BCR-ABL–positive cell line, treatment with TKIs is associated with a transient imbalance between survival and apoptotic pathways, which results in tumor cell apoptosis. It is obvious, therefore, that if survival or antiapoptotic signaling is preserved or enhanced via alternative mechanisms at the time of BCR-ABL kinase inhibition, tumor cells will survive despite effective oncogene inhibition. This model emphasizes the role of those signaling pathways that remain active in LSCs at the time of TKI treatment. It has already been demonstrated that survival signals, such as those mediated by SRC kinases, can remain active following inhibition of BCR-ABL kinase activity by IM in CML cells. A significant part of the current research in the CML community is exploring several signaling pathways for their role in CML LSC survival and is hoped to reveal alternative approaches to eradicate LSCs. Such approaches would likely consist of combining a TKI with a second inhibitor of survival signaling to more effectively shut down survival pathways active in LSCs ( Fig. 2 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree