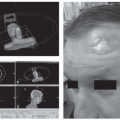
Human Papillomavirus-associated Head and Neck Carcinoma
K. Kian Ang
Erich M. Sturgis
INTRODUCTION
Worldwide, head and neck squamous cell carcinoma (HNSCC) accounts for a relatively small percentage of all cancers, with notable exceptions of high nasopharyngeal cancer incidence in Southeastern China and Southeastern Asia and high oral cavity cancer incidence in Melanesia and South-Central Asia.1 More than 600,000 people were diagnosed with HNSCC worldwide in 2008.2 In the United States, HNSCC accounted for only 3% (about 53,000) of all new cancer cases and only 2% (approximately 12,000) of all cancer deaths in 2010.3 HNSCC affects men 2 to 4 times more commonly than women.
Intensive laboratory and clinical investigations conducted during the past few decades along with technological advances have significantly improved our knowledge of the biology of HNSCC and led to the establishment of new multimodality treatment regimens, improved surgical and radiation therapy methods, and refined management strategies for HNSCC originating at different subsites to optimize tumor control while preserving organs and physiologic functions. As these research efforts have been evolving, the epidemiology and demographics of HNSCC have been changing, particularly during the past decade, mainly because of the appearance and increasing incidence of human papillomavirus (HPV)-related carcinoma.
In this chapter, we address the changing demographics of HNSCC, its diagnosis, and the clinical behavior of HPV-associated oropharyngeal carcinoma, focusing on studies devoted to assessing the discovery of prognostic and predictive biomarkers. Rather than providing an exhaustive review, we chose to present pertinent data to illustrate important discoveries relevant to clinical practice. We also discuss briefly the current research strategies aimed at changing treatment paradigms so as to reduce morbidity while preserving high tumor control rates through well-coordinated clinical trials.
CHANGING EPIDEMIOLOGY OF HEAD AND NECK CANCER
Decline in Tobacco Use and Smoking-related HNSCC
As presented by Giovino in an overview paper and illustrated in Figure 12-1A,4 smoking of manufactured cigarettes accounted for 1% of all tobacco consumed in the United States in the 1880s but this form of use increased progressively to about 80% by 1950, when overall annual tobacco consumption peaked at about 13 lb per person. As evidence mounted regarding the association between tobacco use and lung cancer, cardiovascular disease, and other illnesses, and with the Surgeon General’s warning about the hazards of cigarette smoking, its use diminished consistently since the mid-1960s and dropped to 3.7 lb annually per capita by 2006.4,5 More than half of men and one-third of women in the United States smoked in 1965 but less than a quarter of men and less than one-fifth of women smoked in 2006.4
HNSCC is one of several cancers that are strongly associated with tobacco use.6 As might be expected for diseases with a long initiation phase associated with chronic tobacco exposure, the primary public health goal of reducing cancer incidence by smoking cessation did not begin to occur until the late 1980s.5 Cancer statistics data available at the Surveillance, Epidemiology, and End Results (SEER) website (http://www.seer.cancer.gov/faststats) indeed reveal a steady decrease in the age-adjusted incidence rates of carcinoma of the larynx, the floor of mouth, the gingiva, and most other oral cavity sites since early 1980s Figure 12-1B.7 In contrast, however, the age-adjusted incidence rates of carcinoma of the tongue, the tonsil, and other pharyngeal regions have not decreased over the years and, in contradiction to expectations, even began to increase since approximately the turn of the millennium. These complex trends in oropharyngeal cancer incidence are consistent with the decline of one carcinogenic exposure (smoking) but the emergence of another unrelated etiologic exposure during the same period.
Significant site admixture occurs in the annual U.S. “Cancer Statistics” overview, where all subsites of pharyngeal cancer are listed as a single site, “pharynx” (including not only oropharyngeal cancers but also hypopharyngeal and nasopharyngeal cancers) and where base of tongue (lingual tonsil or oropharyngeal tongue) cancers are often listed collectively with oral (mobile or oral cavity) tongue cancers as the generic site “tongue.”3 More thorough analysis, however, revealed that the age-adjusted incidence of oropharyngeal carcinoma is rising dramatically (about 5% annually) in the United States while that of oral cavity cancer is diminishing (approximately 2% annually).8 Furthermore, the increase in oropharyngeal cancer incidence seems to be primarily represented by middle-aged (40-59-year-old) white men.8 These contrasting changes in trends suggest that exposures to other carcinogens are emerging in the pathogenesis of HNSCC. As summarized below, an increasing body of data now demonstrates
that HPV is the main contributor to the changing demographics and epidemiologic trends of HNSCC. In fact, U.S. and Swedish subsets from population-based registries have demonstrated that over time an increasing proportion of oropharyngeal cancers are HPV positive.9,10,11,12
that HPV is the main contributor to the changing demographics and epidemiologic trends of HNSCC. In fact, U.S. and Swedish subsets from population-based registries have demonstrated that over time an increasing proportion of oropharyngeal cancers are HPV positive.9,10,11,12
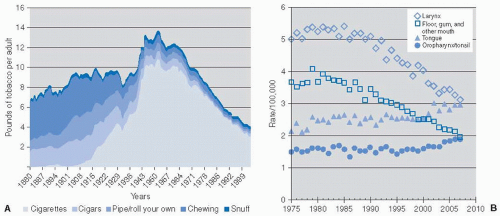 FIGURE 12-1. A: Trends in per capita consumption of various tobacco products in the United States, 1880 to 20044; reproduced with permission. B: Age-adjusted SEER incidence rates for laryngeal cancers, floor of mouth/gingival/other mouth cancer, tongue cancers, and oropharyngeal cancers in the United States. (See color insert) |
Emergence of HPV-Associated Oropharyngeal Carcinoma
The role of some types of HPV, most notably HPV-16 and HPV-18, in the pathogenesis of cancer of the uterine cervix has been recognized for more than two decades (reviewed by zur Hausen13,14). It is now accepted that carcinogenic effects are mediated mainly through two high-risk HPV oncoproteins, E6 and E7, which inactivate the TP53 (also referred to as p53) and retinoblastoma (also referred to as Rb) tumor suppressor gene products, respectively,15,16 and thereby disrupt cell-cycle regulatory pathways.
More recently, several lines of evidence have established the potential of HPVs to induce HNSCC, specifically oropharyngeal cancer. In reviewing epidemiological and experimental evidence, Franceschi et al.17 showed that at least a proportion of cancers of the upper aerodigestive tract harbor relatively high copy numbers of HPV-DNA and transcripts of viral E6/E7 genes, and a clonal association with HPV has been demonstrated in these tumors. A case-control study showed that the presence of HPV DNA in the oral cavity was associated with a higher risk of oral cancer, independent of alcohol and tobacco consumption, with an odds ratio of 3.7 (95% confidence interval [CI], 1.5-9.3). Notably, however, this study suffered from site admixture of oral cavity and oropharyngeal cancers.18 Another study revealed that after adjustment for cotinine levels, the odds ratio for HNSCC in subjects who were seropositive for HPV-16 was 2.2 (95% CI, 1.4-3.4) with no increase in risk observed for other HPV types.19 Remarkably, the odds ratio for oropharyngeal cancer specifically was >14.
A systematic review and meta-analysis revealed that HPV-associated HNSCC is located mainly in the oropharynx and that HPV-16 accounts for the vast majority of these carcinomas.20,21 The prevalence of HPV in oropharyngeal carcinomas has increased steadily in Western countries. Cohorts of patients mainly from the 1990s had prevalence rates of ≤50% whereas more recent series reported rates in the 70% to 80% range.8,10,11,20,21,22,23,24 Chaturvedi et al.8 found in analyzing SEER data that the incidence of all oropharyngeal carcinomas increased significantly between 1973 and 2004, with an annual percentage increase of 0.8 during that time. In a review of tumors from three registries within the SEER, Chaturvedi et al. found HPV prevalence rates increasing over time and were able to estimate that the incidence of HPV-associated oropharyngeal cancer has been increasing over the past two decades, while the incidence of HPV-negative oropharyngeal cancers has declined by approximately half, similar to incidence trends for oral cavity and laryngeal cancer.12 The authors go on to estimate that the number of HPV-positive oropharyngeal cancers will surpass the number of cervical cancers in 2020.12
Interestingly, across the oropharyngeal subsites, the incidence of base of tongue and tonsil carcinomas increased most significantly during this period (with annual incremental rates of 1.3% and 0.6%, respectively) whereas that of pharyngeal wall cancers was stable. In contrast, the incidence of HNSCC originating at anatomical sites unrelated to HPV infection (oral cavity) declined significantly between 1973 and 2004 at a yearly rate of -1.9%. Consequently, the proportion of HNSCC that originated from oropharynx has increased from 18% in 1973 to 31% in 2004. The same phenomenon has been observed in other Western countries such as Sweden.25
HUMAN PAPILLOMAVIRUS
Characteristics of HPV
HPV is a double-stranded DNA virus that infects human epithelia. The >100 existing genotypes are broadly classified as high-risk or low-risk based upon their oncogenic capabilities.14,26
Low-risk types (such as HPV-6 and HPV-11) are associated with both cutaneous and mucosal papillomas, whereas high-risk types (such as HPV-16, HPV-18, HPV-31, and HPV-33) can induce squamous cell carcinomas and are thus termed “oncogenic.”14,26,27 The structure is that of circular viral DNA (approximately 8,000 base pairs) surrounded by viral capsid proteins arranged in a capsule. The approximately 80 genes in the HPV genome are referred to as early (E) or late (L) based upon when during the cell differentiation process they are transcribed and the remaining long control region made up of transcriptional regulators. The early proteins are expressed typically in the basal layer of the epithelia (early in the viral life cycle) and are responsible for gene regulation and replication, whereas the late proteins expressed in more differentiated layers make up the viral capsule,14,26,27 as described below.
Low-risk types (such as HPV-6 and HPV-11) are associated with both cutaneous and mucosal papillomas, whereas high-risk types (such as HPV-16, HPV-18, HPV-31, and HPV-33) can induce squamous cell carcinomas and are thus termed “oncogenic.”14,26,27 The structure is that of circular viral DNA (approximately 8,000 base pairs) surrounded by viral capsid proteins arranged in a capsule. The approximately 80 genes in the HPV genome are referred to as early (E) or late (L) based upon when during the cell differentiation process they are transcribed and the remaining long control region made up of transcriptional regulators. The early proteins are expressed typically in the basal layer of the epithelia (early in the viral life cycle) and are responsible for gene regulation and replication, whereas the late proteins expressed in more differentiated layers make up the viral capsule,14,26,27 as described below.
HPV infects the basal layer of the epithelium through breaks in the stratified (differentiated) layers of the epithelium. How and why these breaks in the epithelium occur differs according to the site of infection. HPV affinity for the basal epithelium of different anatomic sites is, in theory, due to variations in the mechanisms and frequency of access to the basal layer as well as tissue-specific differences in the extracellular matrix and cell surface receptors (principally integrins) at each anatomic site.26 Endocytosis brings the virus into the cell, and subsequently the viral DNA persists as an episome (circular form) in the nucleus.26,27,28 At this point, the episome relies upon the host cellular machinery to replicate along with the host DNA. As the host cell differentiates and moves progressively to the superficial layers, the replicated viral DNA is packaged within viral capsids produced during expression of the late genes. The virus is subsequently released as the superficial epithelial layers are desquamated.
Viral-Mediated Carcinogenesis
The E6 and E7 proteins seem to be critical to the oncogenic potential of HPV because these proteins have the ability to inhibit important tumor suppressor pathways (reviewed by Allen et al.).26 The principal effect of the E6 protein is the binding and enhanced degradation of the p53 protein, thus allowing unregulated cell proliferation. Other p53-independent effects seem to include effects on telomerase. The E7 protein inactivates another tumor suppressor pathway (the Rb pathway) by binding to Rb proteins, thereby allowing release of the transcription factor E2F and subsequent cell proliferation. E7 also seems to have Rb-independent effects by enhancing the transcriptional activity of E2F through direct binding to E2F-DNA complexes as well as by facilitating HPV avoidance of host immune defenses. Although both E6 and E7 are produced by high-risk (oncogenic) and low-risk HPV, differences in the binding affinities with tumor suppressor proteins among HPV genotypes may account for the observed difference in oncogenic potential.29
Most adults have been exposed to HPV, with >80% of women and 60% of men having had a genital infection in their lifetimes.29,30 Lifetime prevalence rates for oral HPV infection are not known, but point prevalence estimates are generally in the 5% to 10% range31 and seem to increase with age and to be higher in men than in women.32 In the largest study to date (>5,500 individuals), oral HPV prevalence, determined by oral rinse and gargle sampling, demonstrated that HPV-62 (followed by types 55, 84, 72, 89, 61, and 6) were the most common low-risk HPV types and HPV-16 (followed by type 66 and less commonly types 51, 39, 56, 52, 59, and 18) were the most common high-risk types.32 In multivariate analyses, these oral infections were associated with older age, male sex, cigarette smoking, and lifetime number of sexual partners (oral sex partners, vaginal sex partners, and any sex partners).32 Most of these infections are cleared,29 and in terms of relative numbers few new cases of cervical, other anogenital, or oropharyngeal cancer are diagnosed annually (<40,000).3
Incorporation of the viral genome into the host genome (rather than remaining in the episomal form) may be a critical step in carcinogenesis.29 Viral DNA insertion tends to occur at certain risk sites (fragile sites) of the host genome, and changes in the viral genome with insertion (particularly interruption of the E2 gene) affect E6 and E7 oncoprotein expression levels.33 Each step raises important prevention opportunities: the possibility of determining who (by genetic variations) is at risk of HPV-associated cancers among those exposed as well as which viral genotypes are the most oncogenic.
Gillison et al. generated evidence in support of the causal relationship between HPV infection and a subset of HNSCC.34 This group of investigators assayed tumor specimens from 253 patients with newly diagnosed or recurrent HNSCC for the presence of the HPV genome by polymerase chain reaction (PCR), Southern blot hybridization, and in situ hybridization; they also sequenced the viral E6 coding region to confirm the presence of tumor-specific viral isolates as well as sequencing exons 5 to 9 of the TP53 gene from 166 tumors. HPV was detected in 62 (25%) of 253 cases. HPV-16 was identified in 90% of the HPV-positive tumors and was localized within the nuclei of cancer cells in preinvasive, invasive, and lymph node disease. Southern blot hybridization patterns were consistent with viral integration. As compared with HPV-negative oropharyngeal cancers, HPV-positive oropharyngeal cancers were less likely to have TP53 mutations. On the basis of available evidence, the International Agency for Research on Cancer concluded in 2007 that HPV type 16 is a cause of oropharyngeal carcinoma (Monograph Volume 90, 2007, which can be accessed from the following website: http://monographs.iarc.fr).
A more recent study compared 28 HPV-16-associated (determined by HPV-specific PCR and fluorescence in situ hybridization [FISH] analysis and positive p16 immunostaining) and 32 HPV-unrelated oropharyngeal carcinomas by comparative genomic hybridization to identify differences in chromosomal and genetic profiles.35 This work showed that HPV-negative tumors had (a) higher numbers of chromosomal alterations (p = 0.03) and amplifications (p = 0.039); (b) more losses at 3p (p = 0.002), 5q (p = 0.03), 9p (p < 0.001), 15q (p = 0.02), and 18q (p = 0.004) and gains/amplifications at 11q13 (p = 0.001); and (c) less often 16q losses (p = 0.02) and Xp gains (p = 0.03). Interestingly, 16q loss, predominantly identified in HPV-related oropharyngeal carcinoma, was a strong determinant of better overall survival (p = 0.008) and disease-free survival (p = 0.01) and none of those patients had a tumor recurrence.
DIAGNOSIS AND CLINICAL FEATURES OF HPV-ASSOCIATED OROPHARYNGEAL CARCINOMA
Histology
No uniform terminology has existed until recently for reporting HPV-associated oropharyngeal carcinoma. Histologically, HPV-associated oropharyngeal carcinomas have been misinterpreted as poorly differentiated carcinomas based on the immature appearance of tumor cells or often described as “basaloid” carcinoma based on the lobular growth of cells with hyperchromatic nuclei and a high nuclear-to-cytoplasmic ratio. A recent study showed that the “basaloid” subtype consists of a mixed group of HPV-positive and HPV-negative carcinomas with very different behaviors.36 The presence of HPV denotes a subset of basaloid squamous carcinoma with a more indolent behavior.36 Therefore,
the recommendation was made to classify this subset of tumors as nonkeratinizing squamous cell carcinoma to avoid confusion and to routinely test all carcinomas arising in the oropharynx for the presence of HPV, as described below.
the recommendation was made to classify this subset of tumors as nonkeratinizing squamous cell carcinoma to avoid confusion and to routinely test all carcinomas arising in the oropharynx for the presence of HPV, as described below.
Methods of HPV Detection
Methods of HPV detection have evolved substantially since a connection between HPV and cancer of the uterine cervix was postulated in the 1980s. Currently available methods include detection of serum antibody against several HPV epitopes, type-specific and consensus (broad spectrum) PCR assays, real-time PCR (RT-PCR) to quantify viral load, type-specific DNA in situ hybridization, and immunohistochemical detection of surrogate markers, particularly a known biomarker of HPV E7 oncoprotein function, the cyclin-dependent kinase inhibitor p16 protein. As recommended at the 2008 meeting of the task force of the National Cancer Institute’s head and neck steering committee, the choice of HPV assay depends on how the information obtained is to be used.37 In short, HPV serology and PCR-based methods have been used mostly for studying the natural history of HPV infection. A weakness of the nonquantitative PCR-based assay is that it cannot distinguish oncogenic virus from biologically irrelevant (trancriptionally inactive) virus. These methods are being refined, and more sophisticated forms may become available for clinical use.
In situ hybridization (ISH) and immunohistochemical (IHC) assays are now widely used for characterizing oropharyngeal carcinoma, and each has strengths and limitations. The presence of punctate hybridization signals within the nuclei of tumor cells is seen only after the HPV DNA has been integrated into the host genome and is thus more biologically relevant.38 The introduction of various signal amplification steps has significantly improved the sensitivity of this technique, even to the point of viral detection down to one viral copy per cell.38 Recent advances also make ISH more practical and cost-effective for everyday use in most diagnostic laboratories that routinely process formalin-fixed and paraffin-embedded (FFPE) tissue blocks. For example, the development of nonfluorescent chromogens now allows display of DNA hybridization using conventional light microscopy, and adaptation of ISH to FFPE tissues has made this technique compatible with standard tissue-processing procedures and amenable to retrospective analysis of archival tissue blocks.37 A type-specific probe is very sensitive for detecting the presence of HPV-16, and a consensus ISH probe can detect 15 HPV types.
Because of its simplicity and ease of interpretation (almost a binomial distribution of staining), the p16 IHC assay has been advocated as a reliable surrogate marker of HPV-associated oropharyngeal carcinoma.39,40 Direct comparison of p16 IHC assay and HPV-16 ISH for relatively large series of HNSCCs reveals a discordance rate of up to 25%. The discrepancies mostly consist of carcinomas that are negative by HPV-16 ISH but are positive by p16. In about half of discrepant cases, p16 expression is attributed to the presence of some other HPV types, as confirmed by wide-spectrum ISH. When E6/E7 mRNA levels were used as a standard of biologically relevant HPV infection, positive p16 was found to be 100% sensitive but only about 80% specific.41 The remaining discordance between HPV ISH and p16 IHC likely reflects the imperfection of p16 as a surrogate marker, as it is also expressed in basaloid carcinomas that are not related to HPV infection (e.g., carcinomas of the breast, lung, and skin).37 Overexpression of p16 could result from perturbations, such as mutational inactivation of the pRB pathway through other mechanisms and perhaps altered methylation or mutation of the p16 gene.42 Overexpression of p16 by IHC has also been documented in HPV-negative nasopharyngeal carcinomas.43
Standardization of assay methods is urgently needed so that reproducible results can be obtained from and compared among diagnostic laboratories. A logical and practical HPV detection strategy is also required to improve consistency in daily clinical practice. An algorithm proposed by Westra at a National Cancer Institute State of the Science Meeting that took place in November 200837 combines the sensitivity and simplicity of p16 IHC with the specificity of HPV ISH. IHC for p16 is recommended as a good first-line assay for eliminating HPV-negative cases from any additional analysis. HPV-16 ISH can be run concurrently with p16 IHC or as a second-line assay after a positive p16 result. Given a specificity approaching 100%, a positive HPV-16 ISH reduces the numbers of false-positive cases by p16 staining alone. A p16-positive/HPV-16-negative result identifies a subset of tumors that qualify for rigorous third-line analysis for other oncogenic HPV types by using a consensus ISH probe that detects 15 other HPV types. This strategy can accurately establish the HPV status of the vast majority of oropharyngeal carcinomas while minimizing the expenditure of resources by limiting the cases requiring multiple assays.
Clinical Features of HPV-Associated Oropharyngeal Carcinoma
HPV-positive oropharyngeal cancers tend to occur in either the tonsils or the tongue base rather than in other oropharyngeal subsites. Numerous case series have established that HPV-positive oropharyngeal cancers have unique demographic, behavioral, and clinical characteristics. A case-control analysis showed a strong association between lifetime sexual behavior and marijuana use for HPV-associated carcinomas, whereas strong trends were observed between consumption of alcohol and tobacco, poor oral hygiene, and HPV-negative cancers.44
Most patients with HPV-positive oropharyngeal carcinoma are middle-aged white men, often with higher socioeconomic status, who consume no or relatively low amounts of tobacco or alcohol.23,44,45,46 As a group, patients with HPV-positive oropharyngeal carcinoma tend to have higher numbers of sexual partners, and in particular, more oral sex partners.44,46,47,48 Though oral mucosal HPV infection is generally thought to be sexually transmitted,32,49 the reason why HPV-related oropharyngeal carcinoma predominantly affects men is not well understood. Possibly, higher HPV viral loads in infected cervical tissues than in penile tissues might result in a higher viral exposure dose and thus increase the probability of contracting HPV infection when performing oral sex on a woman.50
Differences have also been noted in the TNM distribution at the time of diagnosis between HPV-positive and HPV-negative carcinomas. However, published data are not entirely consistent, and the magnitude of these differences varies among series. This discrepancy likely results, to some extent, from the heterogeneous composition of the study population in terms of the primary tumor sites, particularly whether laryngeal cancer was included, and other eligibility criteria for various prospective trials, which limit the generalizability of their results to the oropharyngeal cancer population at large.
Two clinical trials have reported findings from patients with carcinoma at various head and neck sites. The prospective Eastern Cooperative Oncology Group phase II induction chemotherapy trial (protocol ECOG 2399), for example, enrolled patients with stage III or IV squamous carcinoma of the oropharynx or the larynx; tumor specimens were available for assays from 96 patients. More HPV-positive carcinomas were found to have been in earlier T categories (T2, 58% vs. 33%; T3-4, 42% vs. 67%) but in more advanced N categories (N2-3, 66% vs. 50%) compared with HPV-negative tumors.51 The Danish Head and Neck Cancer
Group trials (DAHANCA 6&7) enrolled patients with stage I to IV carcinoma of the larynx, pharynx, or oral cavity and found no significant difference between p16-positive and p16-negative tumors in terms of T category, although p16-positive tumors were more likely to have had nodal involvement than p16-negative tumors (81 of 179 [45%] vs. 192 of 615 [31%]; p = 0.001).52
Group trials (DAHANCA 6&7) enrolled patients with stage I to IV carcinoma of the larynx, pharynx, or oral cavity and found no significant difference between p16-positive and p16-negative tumors in terms of T category, although p16-positive tumors were more likely to have had nodal involvement than p16-negative tumors (81 of 179 [45%] vs. 192 of 615 [31%]; p = 0.001).52
Three clinical trials with large number of patients with oropharyngeal carcinoma had similar patterns of distribution by T and N categories. In the series of 323 patients enrolled in a Radiation Therapy Oncology Group (RTOG 0129) phase III trial,53 HPV-positive carcinomas were on average smaller at diagnosis (T2 34.5% vs. 23.9%; T3 40.8% vs. 36.8%; T4 24.8% vs. 39.3%; p = 0.006) compared with HPV-negative tumors, but no significant difference was found in the distribution by N category between the two groups (N0 7.3% vs. 7.7%; N1 10.7% vs. 20.5%; N2 71.4% vs. 64.2%; N3 10.7% vs. 7.7%; p = 0.46). Notably, however, patients with T1-2 N0-1 disease were not eligible for this trial. Findings from the TAX-324 series of 111 patients were similar to those from the RTOG series, that is, the incidence of T1-2 tumors was higher for HPV-positive carcinomas than for HPV-negative tumors (49% vs. 20%; p = 0.001) but no significant differences were found in nodal status or N category between the two groups.54 In the Trans Tasman Radiation Oncology Group (TROG) trial of 185 cases, however, patients with p16-positive carcinomas had less advanced T category (T1-2 37% vs. 15%; p = 0.001) but more advanced N category (N2-3 86% vs. 65%; p = 0.001).55
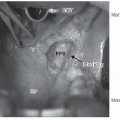
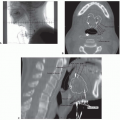
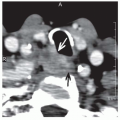
Taken together, these findings show that HPV-positive oropharyngeal carcinomas are generally diagnosed at an earlier T category than are HPV-negative tumors. Findings on the extent of nodal involvement at diagnosis are less consistent among series but a trend seems evident toward HPV-positive oropharyngeal carcinomas presenting at more advanced N categories. Of clinical interest is that involved lymph nodes from HPV-positive carcinoma often appear cystic on imaging.56 This feature is now recognized as a trait of HPV-associated carcinomas, particularly those originating from the tonsil or base of tongue and, therefore, is considered an indication for diagnostic tonsillectomy and multiple base of tongue biopsies when a clinical workup, including examination under anesthesia, fails to detect the primary tumor.
As outlined above, HPV-associated oropharyngeal cancers, as described chiefly in single-institution case series or clinical trials with specific entry criteria, have distinctive demographic, behavioral, and clinical characteristics. Population-level trends in oropharyngeal cancer mirror these characteristics and support HPV as the cause of the national increase in the incidence of oropharyngeal cancer, which is greatest among middle-aged white men.8 Although many behavioral researchers have suggested that the prevalence of oral sex has risen over the past three decades, reliable data on such national trends are lacking. However, it is clear that smoking prevalence has dropped over this period and, as expected, the incidence of tobacco-associated neoplasms have subsequently declined.5,8,57,58,59 National trends also reveal a rising proportion of cancers of the tongue and tonsil presenting as higher-grade tumors, but this is not the case for tumors at other sites.60 In addition, several groups using population-based registries have described dramatic increases in HPV-positive oropharyngeal cancer among archived tumor specimens over time.9,10,12,24,61
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree