Chapter 70 HIV-Related Renal Disease
Diseases of the kidney are generally classified into one of two broad clinical syndromes, acute renal failure (ARF) and chronic kidney disease (CKD), which are distinguished by the rate of change in glomerular filtration rate (GFR). This classification helps in the diagnosis, treatment and prognosis of the underlying disorder. However, these syndromes are not mutually exclusive, and some overlap exists. For example, HIV-associated nephropathy is the single most common cause of CKD in HIV-infected patients but it can present as an ARF syndrome. Similarly, although attention has focused on the potential for antiviral agents to cause ARF, recent reports suggest that some agents may be associated with subacute and/or chronic reductions in renal function. Also, appropriate use of antivirals in CKD is an area of growing importance. The distinction between acute and CKD can narrow the differential diagnosis, but accurate diagnosis can only be made with a detailed history and physical, relevant laboratory data including urinalysis, an assessment of kidney size and structure, knowledge of the nephrotoxic potential of concomitant medications, and often a kidney biopsy. This chapter will update the definitions of the important renal syndromes, review the disorders that commonly occur in HIV, and provide a clinical framework for diagnosis and treatment.
CHRONIC KIDNEY DISEASE
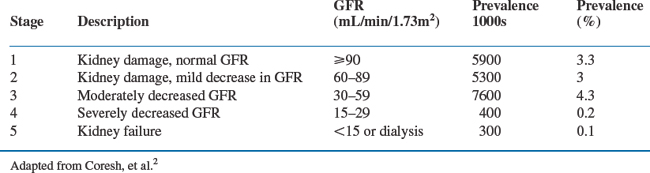
CKD is defined as kidney damage or reduced kidney function lasting longer than 3 months, regardless of diagnosis.1 Abnormal renal pathology on biopsy or a surrogate marker, such as proteinuria or an abnormal imaging study, is the standard for defining kidney damage. Patients with a normal GFR (>90 mL/min per 1.73 m2), or mild reductions in GFR (60–89 mL/min per 1.73 m2) are classified as stage 1 and 2 CKD, respectively, when kidney damage (proteinuria or an abnormal imaging study) is also present. A GFR lower than 60 mL/min per 1.73 m2 defines reduced kidney function. CKD stages 3–5 are classified according to the magnitude of the reduction in GFR, irrespective of the cause of kidney disease or whether proteinuria or an abnormal imaging study can be demonstrated (see Table 70-1(2)). The primary outcome of interest for patients with CKD is the risk of progressing to end-stage renal disease (ESRD) requiring dialysis. But CKD also places patients at risk for other co-morbid conditions, such as hypertension, cardiovascular disease, anemia, and hyperparathyroidism. Finally, impaired renal function places patients at risk for adverse events or drug toxicity caused by improper dosing of drugs that are excreted by the kidneys.
The prevalence of CKD in the United States is alarmingly high, estimated at 19–20 million people.2 The rate of hospitalizations, cardiovascular events and all-cause mortality increases inversely with GFR, reaching three- to sixfold when GFR is reduced to moderate or severe levels.3 Medicare patients with CKD are 5–10 times more likely to die from cardiovascular disease than to reach ESRD.4 These striking epidemiologic data have stimulated several major public health initiatives aimed at increasing awareness of kidney disease so that patients can be identified early in the course of their disease when medical intervention could be more effective. The past several years have witnessed tremendous growth in our understanding of the epidemiology of early stages of CKD, including kidney diseases associated with HIV infection.
The Prevalence of CKD in HIV-Infected Patients
Several studies provide insight into the prevalence of CKD associated with HIV-infection. Fourteen percent of women in the WIHS cohort met criteria for CKD, as defined by two consecutive dipsticks for proteinuria of 1+ or greater (30–100 mg/dL).5 Kidney disease was associated with older age, black race, a history of hypertension or diabetes, lower BMI, and a prior AIDS-defining illness. In an analysis of the HERS cohort, kidney disease was defined as a serum creatinine >1.4 mg/dL or urine dipstick >2+ on any research visit between 1993–97.6 Kidney disease was present in 7.2% of women at baseline, and was three-fold higher compared to controls (seronegative women selected because of high-risk behavior). Similar to associations described in the WIHS cohort, kidney disease in the HERS study was associated with black race and older age. HCV co-infection, injection drug use and HIV viral load were also independent risk factors. After a mean follow-up of 21 months, renal abnormalities were four times more frequent in seropositive women compared to controls (15.6% vs 4.3%). Gupta et al, studying patients in an urban hospital, defined CKD as a doubling of serum creatinine from the time of their initial examination through follow-up visits.7 Two percent of the entire cohort and 9% of black patients met this definition. This is certainly an underestimate because, by today’s standards, a doubling of serum creatinine is an insensitive marker for CKD. Twenty-nine percent of patients had dipstick proteinuria >1+. The prevalence of CKD was reviewed in a clinic, located in an urban medical center. Of 1200 patients, most of whom were black or Hispanics, 9% had an estimated GFR <60 mL/min per 1.73 M2 as determined by the modification of diet in renal disease study (MDRD) formula.8 A high prevalence of hypertension and diabetes was noted in the cohort. Jung et al. also reported a strong association between renal disease (proteinuria) and hypertension in HIV-infected patients. Proteinuria was present in 41% of their hypertensive patients.9
The prevalence of renal disease in HIV-infected patients from developing countries is even more alarming. Olson et al, reporting on the experience of Doctors without Borders, found that 19% of patients in Guatemala city, and, astonishingly, 43% of patients in Arua (Uganda) have estimated GFR values <50 mL/min, by Cockroft–Gault formula, when initiating antiretroviral therapy.10 The high prevalence of kidney disease, particularly in Africa, could be spurious because of calibration differences in measuring serum creatinine. But, it is important to recognize the possibility that this represents the beginnings of a kidney disease epidemic in sub-Saharan Africa, based on the high prevalence of kidney disease in HIV infection and the kidney disease risk associated with black race.
A recent presentation of data from the CDC’s Adult/Adolescent Spectrum of HIV Disease Project followed serum creatinine in ∼10 000 outpatients.11 Their findings were similarly dramatic. MDRD GFR was <60 mL/min per 1.73 m2 in 9% of subjects. GFR was between 60–89 mL/min per 1.73 m2 in 35%. While care must be taken in interpreting the significance of GFR levels between 60–89 mL/min per 1.73 m2, it is reasonable to estimate that as many as 10% of HIV-infected patients of African descent have CKD based on a reduction in GFR. Futhermore, as many as 20–30% may have CKD using proteinuria as the criterion.
ASSESSING KIDNEY FUNCTION IN HIV
Glomerular Filtration Rate
Several equations have been developed to predict GFR more reliably than serum creatinine alone, using best-fit or maximum likelihood mathematical models. These models equate variables associated with muscle mass and creatinine generation to a reference standard for GFR, either the renal clearance of an endogenous marker (a timed urine collection for calculated creatinine clearance) or an exogenous marker (infused iothalamate). The Cockroft–Gault formula and the MDRD equation, shown in Table 70-2, have been validated more than any others. The Cockroft–Gault formula was derived from a cohort of 290 patients and uses age, gender, and weight to predict endogenous creatinine clearance as the reference standard for GFR.12 The MDRD equation was derived from ∼1600 patients with CKD who participated in the NIH-sponsored, multicenter, MDRD study.13 The full equation incorporates several predictor variables and GFR is measured by the clearance of infused iothalamate, the gold standard reference. Several variables have subsequently been dropped from the original equation, resulting in what is now termed the modified MDRD equation. This modification has increased clinical utility of this method without a loss of precision.1
Table 70-2 Comparison of Cockroft–Gault Formula and Modified MDRD Equation
| Cockroft–Gault Formula 140 – Age (yrs) × Weight (kg) (72 × Scr) × (0.85 if female) | MDRD Equation 186 × (Scr)−1.154 × (Age)−0.203 × (0.742 if female) (× 1.21 if African American) | |
|---|---|---|
| Publication | Nephron 1976; 16:31–41 | Levey et al Ann Int Med 1999; 130:461–470 |
| Prediction model | Best fit | Stepwise linear regression |
| Response variable | 24 h urine creatinine clearance | 125I iothalamate clearance |
| Validation | 200 hospitalized patients with wide range of clearance values | 1600 patients with CKD and GFR 20–60 mL/min/1.73 m2 |
| Caveats | Assumes lean body mass; not validated in special populations, including HIV | Not validated for GFR > 60 mL/min/1.73 m2, and in special population, including HIV |
| Recommended uses | Dose adjustment for drugs excreted by kidneys | Reliable estimate of GFR |
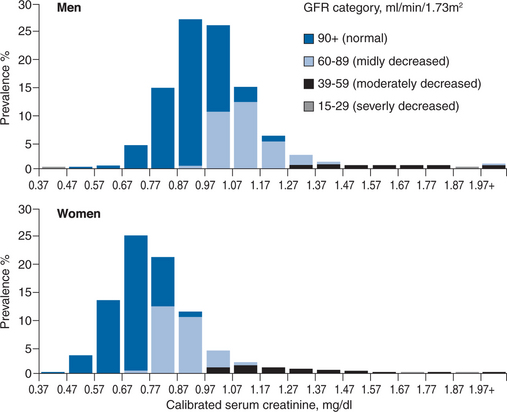
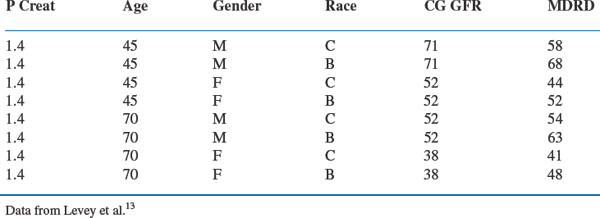
Either equation is superior to serum creatinine alone when evaluating kidney function in clinical practice. The Cockroft–Gault formula has been used to estimate kidney function when studying drug clearance and it remains the standard to use when adjusting drug dose in kidney disease. The MDRD equation is a more accurate measure, especially in the range of 20–60 mL/min per 1.73 m2. The importance of either equation in clinical practice cannot be overstated. As illustrated in Figure 70-1 and Table 70-3, these estimates help identify patients with impaired kidney function when serum creatinine levels are within the upper limits of normal or only mildly elevated. In Figure 70-1, GFR, as measured by the MDRD equation, is superimposed on serum creatinine values obtained in the NHANES III survey.2 A considerable proportion of people have mild to moderate reductions in GFR even when serum creatinine levels are well within the normal range. This is especially true for women. Table 70-3 depicts a wide range of GFRs, depending on age, gender, and race, for individual patients with a serum creatinine of 1.4 mg/dL.13 While there are differences between the MDRD and Cockroft–Gault estimates, they remain more accurate than serum creatinine alone. Far greater differences exist across age, gender, and race, than which of the formulas is used to estimate GFR.
Most healthcare providers recognize the significance of high serum creatinine levels (>2 mg%). Unfortunately, many underestimate the importance of high normal or mildly elevated creatinine levels and fail to recognize that their patients have kidney disease when serum creatinine is between 1 and 2 mg%. The National Kidney Disease Education Program encourages clinical laboratories to include GFR estimates when they report serum creatinine.14 This should increase awareness of CKD so we can identify more patients in the early stages and improve quality of care. These reports are now routine in many laboratories and may soon be mandated by several state health departments.
Proteinuria
Testing for proteinuria in adults is performed using a routine dipstick urinalysis.1 Patients with a positive dipstick (+1 or greater) should undergo confirmation with an untimed, ‘spot’ urine tested for either albumin or protein and creatinine concentrations. Albumin is the more sensitive marker for CKD and is the recommended approach, particularly in diabetics. Medical therapy at the albuminuric stage of diabetic kidney disease improves long-term outcome. If albumin-specific dipsticks or albumin assays are unavailable, testing for total proteinuria is a reasonable alternative. The concentration of albumin or protein in the urine depends on whether the urine is concentrated or dilute, but the amount excreted can be readily interpreted when albumin or protein concentration are normalized for urine creatinine concentration. Usual creatinine excretion is ∼1 g/day. Urinary albumin concentration should not exceed 30 mg/gram creatinine, and urinary total protein concentration should not exceed 200 mg per day. Thus, the urinary protein to creatinine ratio should not be greater than 1:5. A ratio of 1.0 estimates protein excretion at 1 g/day and a ratio of >2 g/day approaches nephrotic-range proteinuria. Because 24 h urine collections are cumbersome for the patient and require an additional office visit, the random urine sample for protein/creatinine ratio has largely supplanted timed collections in clinical practice.
Spectrum of Kidney Disease in HIV
A wide spectrum of kidney diseases has been described in association with HIV. Those commonly confirmed by kidney biopsy are listed in Box 70-1.15 Biopsies are performed in a relatively small proportion of patients even though the prevalence of kidney disease in HIV is high. Therefore, patients undergoing a kidney biopsy represent a selected sampling group, usually with a more rapid progression. Their kidney disease is often unexplained or they have a remarkable clinical presentation, such as heavy proteinuria, an active urinary sediment (proteinuria, hematuria, and red blood cell casts), prolonged ARF or CKD that progresses to renal failure over a relatively short period of time. The frequency of fulminant renal failure in HIV infection has decreased over time, probably due to the widespread use of HAART.16 As a result, many fewer biopsies are performed and kidney disease in these patients is often attributed to coexisting hypertension or diabetes, and a definitive diagnosis is not made.
BOX 70-1 Kidney Disease in HIV: Summary of Biopsy Reports
Adapted from Balow JE. Nephropathy in the context of HIV infection. Kidney Int 67:1632–3, 2005.
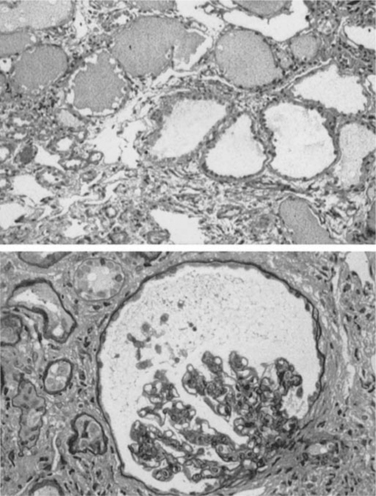
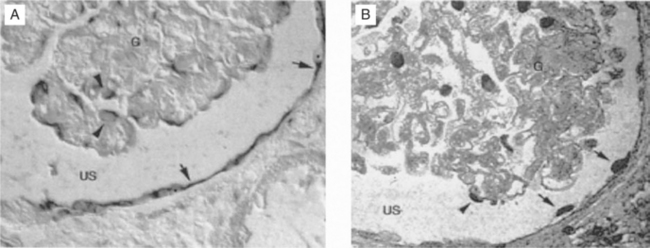
HIV-associated nephropathy (HIVAN) is the single most common cause of CKD found by biopsy in patients with HIV infection.17 It is defined morphologically by collapse of the glomerular capillary tuft, glomerulosclerosis, and microcystic tubulointerstitial disease18 (Fig. 70-2). HIVAN’s association with black race is striking. In biopsy reports throughout North America, Europe, and Asia19,20 over 90% of affected patients are of African descent. This racial predilection accounts for differences in prevalence reported from individual centers. When reports of renal disease in HIV-1-infected subjects come from mainly Caucasian populations, such as reported in studies from San Francisco, Italy, Asia and northern Europe, HIVAN is not a frequent cause of CKD.21–24 When blacks comprise a large proportion of the population under study, as in reports from New York, Washington, DC, Miami, and the African or Afro-Caribbean communities of Paris and London, HIVAN is by far the most common cause of renal disease. It is second only to sickle cell-associated renal disease in its racial clustering in blacks. HIVAN is now the third leading cause of ESRD in blacks between the age of 20 and 64 years, more common than lupus nephritis, polycystic disease, or primary glomerulonephritis.25 A better understanding of the pathogenesis and genetic basis for HIVAN promises to uncover important information about renal susceptibility genes in patients of African descent.26
The characteristic clinical presentation of HIVAN is heavy proteinuria, often in the nephrotic range, with varying degrees of renal insufficiency in an HIV-infected patient of African descent. Renal ultrasound examination often shows normal to large kidney size. The predictive value of these clinical characteristics in distinguishing HIVAN from other forms of glomerulonephritis is limited, and numerous reports confirm that the clinical impression is not always borne out by the biopsy findings.27,28 Prior to the HAART era, the clinical course was marked by progression to renal failure requiring dialysis within weeks to a month, but the natural history has changed dramatically. Now, patients who are receiving antiviral therapy have a more indolent course, with mild to moderate proteinuria and an impaired but stable GFR. In this clinical form, HIVAN may be contributing to more cases of CKD than has been appreciated but it remains undiagnosed in the absence of a definitive kidney biopsy.
Experiments in a murine model of disease support a direct role for HIV-1 in disease pathogenesis. Mice of the TG26 line contain copies of the HIV-1 proviral DNA pNL4-3d1443, generated by deletion of a 3-kb SphI/BalI fragment within pNL4-3 spanning the gag and pol genes. The construct, under the transcriptional control of the native promoter, the long terminal repeat (LTR), is noninfectious and nonreplicating.29 Heterozygote mice develop kidney disease morphologically and functionally indistinguishable from HIVAN in man.30 Cross-transplantation experiments prove that transgene expression is required for disease to develop. HIVAN appears in kidneys transplanted from transgenic mice to normal littermates, but does not develop when normal kidneys are transplanted into a transgenic mouse.31 Renal transgene expression in glomerular epithelial (podocytes) and tubule epithelial cells precedes the development of nephropathy31 (Fig. 70-3). Following transgene expression, cells undergo dedifferentiation and proliferation. Several viral genes, have been implicated in the pathogenesis, particularly the Nef and Vpr genes.32–34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree