History of Immunology
Steven Greenberg
INTRODUCTION
There comes a time during every argument at which the two opposing parties reach a critical juncture: either resolution or impasse. Variations on this essentially Socratic theme have played out in all spheres of human intellectual activity. The dialectic of science ranges from incremental and relatively harmonious shifts in key, to a few abruptly dissonant ones, taking the form of what Thomas Kuhn would refer to as “paradigm shifts.”1 Such is the case with immunology, a field distinguished by more than its fair share of paradigm shifts. Arguably, its first dialectic, between the “cellularists” and the “humoralists,” did not result in an early synthesis, but was characterized by partisan and often entrenched positions. Ultimately, the two parallel paths of cellular and chemical immunology converged, but it was not until the latter half of the 20th century that the two paths became one. How the paths were forged in the first place was an amalgam of the cultural institutions of the time, the creative output of the scientists themselves, and the imperatives of devising effective strategies to combat infection and contagion.
ANTECEDENTS TO THE GERM THEORY OF DISEASE
Ancient Theories of Disease Causation
Religious beliefs in ancient Greece drew contrasts between the sacred or the pure (katharos) and the polluted (miaros).2 Pollution, or miasma, was blamed for many ancient transgressions, from the petty and personal, to the gravest, most famously embodied in the Oedipus myth. To remove the stain of miasma, the transgressor must undergo rites of purification (catharsis). To the ancient Greeks in the age of Homer, these were deeply ingrained beliefs that were essentially religious in nature. Because miasma was viewed as a source of suffering, it is not surprising that miasma was implicated in disease, as described by Hippocrates in his treatise “On Air, Water, and Places” in which miasma was associated with “unhealthy vapors.”3,4 Hippocrates is credited with being the first to recognize the potential of disease to arise from the environment and not as a result of religious superstition. Mal aria, which is Old Italian for “bad air,” was one of many diseases thought to be caused by miasma. The concept that miasma was the source of disease persisted through the millennia and was a leading theory of how contagious diseases were transmitted up until the time of Pasteur.
Much of what we know about the medicine of ancient Greece is codified in The Hippocratic Corpus, a collection of more than 60 volumes of text. Its authorship is disputed, but it is generally recognized as a compilation of works by Hippocrates himself as well as his students and intellectual heirs. One of his students, as well as son-in-law, Polybus, was credited as the author of De Natura Hominis (On the Nature of Man), the earliest known text describing the ancient Greek conceptual basis for disease pathogenesis, as embodied in the four humors: black bile, yellow bile, phlegm, and blood.5
The body of man has in itself blood, phlegm, yellow bile and black bile; these make up the nature of his body, although these he feels pain or enjoys health. Now he enjoys the most perfect health when these elements are duly proportioned to one another in respect of compounding, power and bulk, and when they are perfectly mingled. Pain is felt when one of these elements is in defect or excess, or is isolated in the body without being compounded with all the others.
The Greek view that disease arose from an imbalance of the four humors did not supersede the miasma theory of disease, but was rather a more general view of disease causation, compared with the subset of apparently communicable diseases best explained by miasma.
The Romans developed and refined Greek concepts of disease. Marcus Terentius Varro (116 to 27 bce), referred to as “the most learned of all Romans” by the Roman rhetorician Quintilian,6 was a prolific Roman scholar, estimated to have written more than 600 volumes. During the civil war of the first century, he served as Pompey’s legate in Spain and fought at Pharsalus against Caesar but ultimately reconciled with Caesar, who appointed him director of the public library in 47 bce. Varro is perhaps best known for his only complete extant work, Rerum Rusticarum Libri Tres (On Agricultural Topics), in which he so presciently anticipated the existence of disease-causing microbes that seemed to Varro to be the immediate cause of diseases7:
Precautions must also be taken in the neighbourhood of swamps, both for the reasons given, and because there are bred certain minute creatures which cannot be seen by the eyes, which float in the air and enter the body through the mouth and nose and there cause serious diseases.
Thus, Varro provided a mechanistic basis for disease that was consistent with the prevailing belief of miasma as the source of illness.
The Greek medical tradition was carried on for generations and was ultimately passed on to Claudius Galinus
(Galen), the Greek expatriate who was its greatest explicator. Galen was born in Pergamum in Asia Minor in 130 ce. After beginning his medical training in Pergamum at the behest of his father, he traveled widely in pursuit of “postgraduate” medical training in Smyrna, Corinth, and Alexandria. He returned to Pergamum and practiced surgery on gladiators, which provided a unique opportunity to deepen his knowledge of human anatomy and perfect his surgical technique.8 Following an outbreak of plague among the Roman troops in Aquileia in 168 ce, he was summoned by the Emperor Marcus Aurelius and was appointed personal physician to his son, Commodus.8 Galen’s view of medicine was based on Hippocrates’ Corpus. His output was prodigious more than any other ancient author of medical texts. He distinguished symptoms from diseases and offered explanations of the former that were consistent with his interpretations of disease pathogenesis; thus, tertian fever was the result of an “imbalance of yellow bile,” quartan was caused by “too much black bile,” and quotidian by “an excess of phlegm.” Vomiting was viewed as the body’s attempt to expel poisons, and the prescription of bleeding was to rid the body of “corrupt humors.”9 Galen’s view of medicine remained the dominant one until the 17th century.
(Galen), the Greek expatriate who was its greatest explicator. Galen was born in Pergamum in Asia Minor in 130 ce. After beginning his medical training in Pergamum at the behest of his father, he traveled widely in pursuit of “postgraduate” medical training in Smyrna, Corinth, and Alexandria. He returned to Pergamum and practiced surgery on gladiators, which provided a unique opportunity to deepen his knowledge of human anatomy and perfect his surgical technique.8 Following an outbreak of plague among the Roman troops in Aquileia in 168 ce, he was summoned by the Emperor Marcus Aurelius and was appointed personal physician to his son, Commodus.8 Galen’s view of medicine was based on Hippocrates’ Corpus. His output was prodigious more than any other ancient author of medical texts. He distinguished symptoms from diseases and offered explanations of the former that were consistent with his interpretations of disease pathogenesis; thus, tertian fever was the result of an “imbalance of yellow bile,” quartan was caused by “too much black bile,” and quotidian by “an excess of phlegm.” Vomiting was viewed as the body’s attempt to expel poisons, and the prescription of bleeding was to rid the body of “corrupt humors.”9 Galen’s view of medicine remained the dominant one until the 17th century.
Early Concepts of Immunity
The term “immunity” itself is derived from the Latin practice of “exemption” from taxes or public service that normal citizens had to discharge, a favor bestowed by the emperor to meritorious individuals or entire communities.10 However, the concept of immunity dates back at least as far as Thucydides, who described the plague of Athens of 430 bce that was responsible for the death of more than a quarter of the Athenian population11:
Yet it was with those who had recovered from the disease that the sick and the dying found most compassion. These knew what I had from experience and had now no fear for themselves; the same man was never attacked twice-never at least fatally.
The traditional, religious view of the plague was that it is the work of Apollo who was held responsible for earlier plagues (eg, the plague on the Greek army in Troy because the Greek general Agamemnon abducted the daughter of Apollo’s priest, Chryses). The religiously inclined could take some refuge in appealing to Apollo’s son, Asklepios, who was revered as the god of healing, as were his daughters Hygeia (Hygiene), Iaso (Remedy), Akæso (Healing), Aglæa (Healthy glow), and Panakeia (Cure-all). In contrast, Thucydides characteristically did not offer facile religious explanations for sickness or recovery. In fact, he described the futility of the Athenians’ plight in stark terms11:
Neither the fear of the gods nor laws of men awed any man, not the former because they concluded it was alike to worship or not worship from seeing that alike they all perished, nor the latter because no man expected that lives would last till he received punishment of his crimes by judgment.
It seemed inevitable that in seeking an explanation for why some developed disease and others did not, many others would rely on a moralistic or religious view. In a remarkable passage from Galen’s On the Different Types of Fever, not only does he set forth an explanation of how disease is transmitted through the air, and using the same term as Fracastoro would, “seeds,” some 1300 years later, but he also blames a licentious lifestyle for susceptibility to the plague:
Suppose, for example, that the circumambient air carries certain seeds of plague, and that of the bodies which share [breathe] it, some are full of various residues which are soon to become putrefied in themselves, while others are clean and free of such residues. Assume also that in the former there is a general blockage of their pores, a so-called plethora, and a life of ease devoted to gluttony, drink and sex, with all their necessarily concomitant digestive disorders. The others, which are clean and lack these residues, as well as being fine in themselves, have all a wholesome transpiration through pores that are neither blocked nor constricted; they take appropriate exercise and lead a temperate life. Assuming all this, which of these bodies is most likely to be affected by the rotting air they inspire?
This passage has been analyzed extensively by Nutton, who questioned the extent to which “seed” is used metaphorically12; if so, it is particularly apt.
Religious explanations for disease and immunity persisted throughout history. Particularly during the growth of Christianity during the Middle Ages, disease and sin were linked, though not inextricably; the great theologian Thomas Aquinas provided this distinction between sin and other causes of diseases13:
… we need to consider that sin consists of a disorder of the soul, just as physical disease consists of a disorder of the body. And so sin is a disease of the soul, as it were, and pardon is for sin what healing is for disease.
Yet for many, a disease as dire as the plague would continue to be viewed as divine retribution for sin; for others, it was the result of astrological phenomena, while for still others, it was the result of a “conspiracy plotted by Jews to poison wells.”14 With the exception of the latter, which at least offered a proximate physical cause of disease, regardless of the lack of evidence, there was an abstract quality to these explanations that were shrouded in belief and superstition but lacking in substance. Further theories of disease pathogenesis would have to await the 16th century.
Fracastoro’s Seeds
Girolamo Fracastoro (1478 to 1553) was an Italian who would have met most definitions of a Renaissance scholar: an accomplished physician, poet, mathematician, botanist, and astronomer. Educated by his father in Verona, and later at the University of Padua, he became an instructor in logic at the University of Padua in 1501 and in anatomy in 1502. He left Padua in 1508 and returned to Verona, where he
dedicated himself to his studies and his medical practice. In 1546, he proposed that disease was caused by seminaria (“seeds”) that could be transmitted by three ways: direct contact from one person to another; through “fomites,” or articles of clothing or dirty linen; and through the air. Although Nutton12 has pointed out that Galen and Lucretius also used the term “seeds” to describe the transmission of illness through the air (see previous discussion), Fracastoro was the first to make them the focal point for disease transmission and to describe their predilection for certain organs. Like the 10th century Arab physician Rhazes, who believed smallpox to have an affinity for blood, and specifically for the traces of menstrual blood that were believed to taint everyone in utero, as later suggested by Avicenna, Fracastoro offered the following explanation for immunity to smallpox: Following infection by smallpox seminaria, the menstrual blood would putrefy, rise to the surface, and force its way out via the smallpox pustules.15 “Hence when this process has taken place, the malady usually does not recur because the infection has already been secreted in the previous attack.”
dedicated himself to his studies and his medical practice. In 1546, he proposed that disease was caused by seminaria (“seeds”) that could be transmitted by three ways: direct contact from one person to another; through “fomites,” or articles of clothing or dirty linen; and through the air. Although Nutton12 has pointed out that Galen and Lucretius also used the term “seeds” to describe the transmission of illness through the air (see previous discussion), Fracastoro was the first to make them the focal point for disease transmission and to describe their predilection for certain organs. Like the 10th century Arab physician Rhazes, who believed smallpox to have an affinity for blood, and specifically for the traces of menstrual blood that were believed to taint everyone in utero, as later suggested by Avicenna, Fracastoro offered the following explanation for immunity to smallpox: Following infection by smallpox seminaria, the menstrual blood would putrefy, rise to the surface, and force its way out via the smallpox pustules.15 “Hence when this process has taken place, the malady usually does not recur because the infection has already been secreted in the previous attack.”
Fracastoro’s seminaria theory remained influential for nearly three centuries, in many ways serving as an early template for the germ theory of disease.
THE GERM THEORY OF DISEASE AND DEVELOPMENT OF VACCINES
Until the mid-19th century, the Galenic view of disease origins was the dominant one. Not only was the etiology of diseases misunderstood but also was the origin of life itself. The theory of spontaneous generation, which arose from Aristotle, held that life originated spontaneously from inanimate matter. The first experimental evidence against spontaneous generation came from Franceso Redi, the head physician in the Medici court, who in 1668 provided early evidence against the theory.16* Nevertheless, no overarching theory was proposed to replace it. Neither the mindset nor the necessary technology were available until the latter part of the 17th century, when an apprentice in a dry goods store, Anton van Leeuwenhoek, began a lifelong obsession with grinding the perfect lens. Leeuwenhoek’s lenses were tiny but were ground with high degree of curvature, enabling him to visualize the hitherto undiscovered word of microbes. On September 17, 1683, Leeuwenhoek wrote a letter to the Royal Society, which was the first description of living, motile bacteria obtained from the plaque of his own teeth.17 Leeuwenhoek was not the first to build a microscope (which was used by, among others, Redi), but his was far superior to existing multilensed or compound microscopes. Other scientists of the time, notably Robert Hooke, also observed microorganisms, and it was Hooke who was the first to publish the first image of a microorganism (the fungus Mucor) in 1665.18 Some 150 years later, Dutrochet and then later Schwann, Schleiden, and Virchow, taking advantage of the microscopes of the their time, advanced the concept that “all living things are composed of cells and cell products.”19 Virchow took this one step further by declaring omnis cellula e cellula or “all cells develop only from existing cells.” Whether Virchow rejected the germ theory or not is a matter of debate, but it is more likely that Virchow’s underlying emphasis was not on external causes, but disease mechanisms, as he wrote in 1885: “First the discovery of the parasite, then the investigation of its etiology, then the question: how does it give rise to the disease.”20 Although it is hard to escape the possibility that a certain degree of professional jealousy may have played a role in Virchow’s refusal to embrace the germ theory of disease, his viewpoint is one of but many examples of apparently strict dichotomies in science that would ultimately undergo revision and later synthesis. This is a theme that was to be recapitulated many times in the history of immunology.
The Conceptual Basis for the Germ Theory of Disease
Between Leeuwenhoek’s technical breakthroughs in lens design in the late 17th century and the work of Pasteur and Koch in the late 19th century, several individuals endorsed Fracastoro’s “seed” theory, which gained new relevance when bacteria were first visualized. Among these was Jacob Henle, a German pathologist who was later to become Koch’s teacher. Henle wrote a treatise that not only laid out the germ theory of disease in great detail but also arguably articulated an early version of what would later be known as “Koch’s postulates”21: “Before microscopic forms can be regarded as the cause of contagion in man, they must be constantly found in contagious material. They must be isolated from it and their strength tested.” However, Henle’s essay was a theoretical one and Henle himself never provided any experimental evidence in support of it. In the same year, decades before Pasteur and Koch would even begin to describe the germ theory of disease, Henry Holland, a Scottish-trained physician to Queen Caroline, who traveled extensively and was acquainted with the scientific luminaries of the time, including Davie, Gay Lussac, Berthollet, and Laplace, wrote a treatise in which he stated,22
The question is, what weight we may attach to the opinion that certain diseases, and especially some of epidemic and contagious kind, are derived from minute forms of animal life, existing in the atmosphere under particular circumstances; and capable, by application to the lining membranes or other parts, of acting as a virus on the human body.
In a footnote, he cited others, including Kircher, and particularly Johannes Nyander, who wrote nearly a century earlier23:
… it may be an easy matter for very minute insects to be the causes of diverse contagious diseases,” of which he included plague, measles, smallpox, and syphilis.
Experimental Evidence for the Germ Theory of Disease
The honor of the first experimental demonstration for the germ theory of disease may belong to two students of Francesco Redi. In 1687, soon after Redi had offered proof against spontaneous generation, two of his students, Bonomo and Cestoni, went on to observe the causative agent of scabies using the newly developed microscope and were able to transfer disease from person to person.24 Perhaps the first demonstration of the bacterial pathogenesis of diseases in animals and humans was by Casimir Davaine, a French scientist who provided essentially the same evidence that Pasteur and Koch would years later that anthrax was caused by bacteridies. In 1865, Davaine was awarded the Prix Bréant by the Académie des Science for his work.25
Davaine’s compatriot, Louis Pasteur, was the consummate experimentalist. A chemist by training, his interest in infectious disease began with his study of fermentation. He made important contributions to the science of fermentation and his work led to many practical benefits to the beer and wine industry of France. His interest in microbes began with his speculation that the same type of microbe required for fermentation was likely responsible for transmitting disease. In 1865, he was asked to investigate a disease called pébrine that affected the silk worm industry. Within a year, Pasteur had established that pébrine was caused by a microbe, which provided further proof for the germ theory of disease. Some 14 years later, his expertise was again sought out of economic interests, in this case by farmers whose poultry stocks were diminished by chicken cholera. In a famous example of scientific serendipity, his assistant, Charles Chamberland, failed to inoculate chickens with cultures of chicken cholera bacilli, as instructed by Pasteur, but instead went on vacation. Upon returning several weeks later, he inoculated chickens with the old bacterial cultures, but the chickens didn’t die as expected. Rather than disregard the experiment as a failure, “chance had favored the prepared mind” of Pasteur, who had his assistants inject fresh cholera into the same hens that had previously been injected; now none of the hens became ill. Pasteur had surmised that the bacterial cultures had become weakened by extended culture.26 Thus began the use of attenuated strains of microbes to immunize against disease,27 and the birth of the science of vaccination. The term “vaccine,” derived from the Latin vaccus for cow, was, coined by Pasteur in honor of Jenner. Attenuation as a strategy of developing vaccines would prove to be enormously valuable, leading to development of the first rabies vaccine by Pasteur himself, a vaccine against the viral causative agent of yellow fever by Theiler,28 and the Bacillus Calmette-Guérin vaccine at Pasteur’s institute.29
Although Robert Koch is credited as the originator of “Koch’s Postulates,” it may come as a surprise that the essence of the postulates were first formulated by Koch’s teacher, Jacob Henle (see previous discussion) and his contemporary, Edwin Klebs.30 However, neither Henle nor Klebs applied their theories to any practical benefit, which is why Koch received credit for the postulates. Koch was the first to articulate then systematically apply them to prove that Bacillus anthracis was the causative agent of anthrax. Koch was a country physician living in Prussia, whose scientific career began unceremoniously with a gift of a microscope from his wife.31 His first series of investigations began with observing the blood of a dead cow that succumbed to anthrax. Confirming the observation of Davaine and others before him, he observed filamentous bacteria in the blood. Not content merely with the observation, he began a series of technically challenging experiments, necessitating the development of many novel techniques used in microbiology laboratories even today, such as the use of solid medium to grow individual clones or colonies of bacteria. He proved that the filamentous bacteria were present only in infected animals and were capable of reproducing the disease when injected into healthy animals. This was the first systematic application of the eponymous postulates, which has since become the sine qua non of disease causation by infectious agents. His work was published in 1876,32 the first of many groundbreaking publications. Koch’s most profoundly important contribution to medicine was the discovery of the causative agent of tuberculosis. The lecture at which he announced his findings, on March 24, 1882, described later by Ehrlich as “the most important experience of his scientific life,” is considered by many to be the single most important lecture in medical history. Koch described the invention of novel staining methods to detect the tubercle bacillus and presented tissue dissections from guinea pigs that were infected with tuberculous material from the lungs of infected apes and humans who had died from the disease.33 For “his investigations and discoveries in regard to tuberculosis,” Koch was awarded the Nobel Prize in 1905.
The Unhealthy Rivalry Between Pasteur and Koch, and its Lasting Effects
The Franco-Prusssian war of 1870, the culmination of years of tension between France and Prussia, resulted in a Prussian victory and unity among the German states under King Wilhelm of Prussia. The Treaty of Frankfurt left a unified Germany the city of Strasbourg as well as possession of Alsace and the northern part of Lorraine, which was thought to contribute to further resentment of the Germans by the French and public support for World War I. It is against this backdrop that the relationship between Pasteur and Koch must be viewed. Koch had served in the Prussian army, and Pasteur’s son was a conscript fighting for the French. Furthermore, there was intense professional rivalry between the two, especially over their work on anthrax pathogenesis. According to a letter from Charles Ruel, former privat docent at the University of Geneva, Koch was in the audience when Pasteur spoke on attenuation and vaccination at the fourth International Congress of Hygiene and Demography held in Geneva in September 1882. Pasteur spoke repeatedly about
German collected works (recueil Allemand). According to the letter34:
German collected works (recueil Allemand). According to the letter34:
Koch and his friend Prof. Lichtheim, were sitting side by side; they knew French only imperfectly and both mistook the word pride (orgueil) for collection (recueil). They felt their self-respect profoundly wounded and interpreted the words German pride as a grave insult.
This is but one ironic example of the level of rancor and misunderstanding between the two great men. It is said that Pasteur and Koch underwent some form of reconciliation later in their lives. Regardless, the aftermath of their rivalry, in many ways personifying the bitter relations between France and Germany, had a lasting effect on the evolution of the nascent field of immunology. In the years to come, the intellectual heirs of Pasteur and Koch would reenact the lifelong competitive tensions that characterized their relationship.
The Germ Theory of Disease: A Summation
In many ways, the “germ theory of disease” really did not begin with Pasteur and Koch, but rather by their predecessors, Henle, Klebs, and Davaine, who in turn owed credit to Fracastoro, and ultimately to Galen and Varro, some 1,600 years earlier. What enabled Pasteur and Koch to firmly establish the germ theory of disease began as a thought process that over time became distilled to something tangible: First, the idea that invisible “seeds” might propagate disease; second, the advent of an optically superior microscope, by Leuwenhoek, which enabled scientists their first glimpses at the “minute creatures” postulated by Varro; third, the growing evidence against spontaneous generation that began with Redi, thus opening the door for a new theory of disease; and finally, the inductive genius and careful experimental techniques of Pasteur and Koch.
The Long History of Vaccination: Success and the Unprepared Mind
Vaccination did not originate with Pasteur; its practice had been carried out for centuries without any fundamental understanding of its basis. Probably the earliest recorded example of intentionally inducing immunity to an infectious disease was in the 10th century in China, where smallpox was endemic.35 The process of “variolation” involved exposing healthy people to material from the lesions caused by the disease, either by putting it under the skin, or, more often, inserting powdered scabs from smallpox pustules into the nose. Variolation was known and practiced frequently in the Ottoman Empire, where it had been introduced by Circassian traders in the 17th century.35 Unfortunately, because there was no standardization of the inoculum, variolation occasionally resulted in death or disfigurement from smallpox, thus limiting its acceptance. Variolation later became popular in England, mainly due to the efforts of Lady Mary Wortley Montague. Lady Montague was the wife of the British ambassador to the Ottoman court who herself had contracted a severe case of smallpox. While in Istanbul, Lady Montague observed the practice of variolation. Determined not to have her family suffer as she had, she directed the surgeon of the embassy to learn the technique and, in March 1718, to variolate her 5-year-old son. After her return to England, she promoted the technique and had her surgeon variolate her 4-year-old daughter in the presence of the king’s physician. The surgeon, Charles Maitland, was given leave to perform what came to be known as the “Royal Experiment,” in which he variolated six condemned prisoners who later survived. By these and other experiments, the safety of the procedure was established, and two of the king’s grandchildren were variolated on April 17, 1722. After this, the practice of variolation spread rapidly throughout England in the 1740s and then to the American colonies.35,36
It is difficult to say what influence the English country physician Edward Jenner (1749 to 1823) had on Pasteur’s later discovery of attenuation of bacterial cultures and its application to vaccination. Regardless, it is fair to say that Jenner had a major influence on public health, as he was the first to publish the development and use of a safe alternative to variolation.37 Although Jenner is rightly celebrated for his development of cowpox as a safe vaccine for smallpox, he was not the first to make use of a relatively nonpathogenic virus to induce immunity. Twenty years earlier, Benjamin Jesty, an English farmer, inoculated his wife and two sons with material taken from the cowpox lesion of the udder of a cow in his neighbor’s herd.36 In 1796, Jenner inoculated James Phipps, an 8-year-old boy, with material obtained from a cowpox lesion that appeared on the hand of a dairymaid (Fig. 2.1). Six weeks later, he inoculated the experimental subject with smallpox without producing disease. Further studies by Jenner established the efficacy of his vaccination procedure. For this feat, Jenner received a cash prize of £30,000 and election to nearly all of the learned societies throughout Europe.38
EMERGENCE OF IMMUNOLOGY AS A DISCIPLINE
The Cellularists versus the Humoralists
The international renown of Pasteur and Koch led to the establishment of research institutes that bore their names. Many talented young scientists were drawn to these institutes whose research missions should have been complementary, but were not, and the partisan battle that began with Pasteur and Koch would soon be reenacted on a larger scale.
Metchnikoff and the Birth of Cellular Immunology
Elie Metchnikoff (1845 to 1916), an ambitious student at the University of Kharkoff (“I have zeal and ability, I am naturally talented—I am ambitious to become a distinguished investigator”),31 borrowed a professor’s microscope and began a lifelong quest to understand the cellular basis for immunity. A comparative zoologist by training, his early academic focus was on understanding the development of metazoans. Heavily influenced by Darwin’s publication of The Origin of Species in 1859,39 he viewed early embryologic development as a competition among specialized cell
types.40 Ontogeny was seen as a set of “interactions of cell lineages with each other to limit self-replication by any one component in favour of the interests of the organism as a whole.”40 He focused his interest on an amoeboid marker cell of the mesoderm, which was dubbed “phagocyte” (devouring cell) by a contemporary of Metchnikoff’s, the Viennese zoologist, Carl Claus. In a justly famous passage from Olga Metchnikoff’s biography of her husband, she describes the observation that became the defining moment of his scientific career41:
types.40 Ontogeny was seen as a set of “interactions of cell lineages with each other to limit self-replication by any one component in favour of the interests of the organism as a whole.”40 He focused his interest on an amoeboid marker cell of the mesoderm, which was dubbed “phagocyte” (devouring cell) by a contemporary of Metchnikoff’s, the Viennese zoologist, Carl Claus. In a justly famous passage from Olga Metchnikoff’s biography of her husband, she describes the observation that became the defining moment of his scientific career41:
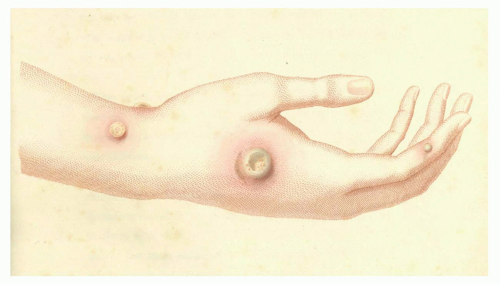 FIG. 2.1. Cowpox Pustule on the Arm of Sarah Nelmes. Reprinted with permission from Jenner37; courtesy of Dr Jenner’s House: Birthplace of Vaccination, Gloucestershire, UK. |
One day when the whole family had gone to a circus to see some extraordinary performing apes, I remained alone with my microscope, observing the life in the mobile cells of a transparent star-fish larva, when a new thought suddenly flashed across my brain. It struck me that similar cells might serve in the defense of the organism against intruders. Feeling that there was in this something of surpassing interest, I felt so excited that I began striding up and down the room and even went to the seashore in order to collect my thoughts. I said to myself that, if my supposition was true, a splinter introduced into the body of a star-fish larva, devoid of blood-vessels or of a nervous system, should soon be surrounded by mobile cells as is to be observed in a man who runs a splinter into his finger. This was no sooner said than done. There was a small garden to our dwelling, in which we had a few days previously organised a “Christmas tree” for the children on a little tangerine tree; I fetched from it a few rose thorns and introduced them at once under the skin of some beautiful star-fish larvae as transparent as water. I was too excited to sleep that night in the expectation of the result of my experiment, and very early the next morning I ascertained that it had fully succeeded. That experiment formed the basis of the phagocyte theory, to the development of which I devoted the next twenty-five years of my life.
Contrary to popular belief, Metchnikoff was not the first to visualize and describe phagocytosis, nor was he the first to suggest that it played a role in host defense. In a historical recount of the history of phagocytosis,42 Stossel argues that a Lutheran pastor, Johann August Ephraim Goeze, was the first to describe phagocytosis by microscopic observations of cells derived from hay infusoria in 1777. Much later, German pathologists of the mid-19th century, including Lieberkühn, Henle, and Vogel, drew connections between “pus corpuscles” of wounds and blood corpuscles.42 Others at the time, particularly the English physicians William Addison and Augustus Waller, observed leukocyte migration through capillaries in response to local injury, and Ernst Haeckel, a German marine biologist who was later to oppose Metchnikoff in an early theory of gastrulation, described molluscan leukocytes ingesting India ink particles in 1862.43
A handful of scientists of the time made the conceptual link between phagocytosis and host defense, and Metchnikoff himself cites a few: “When (the phagocytosis theory) is once firmly established, it will be time enough to determine each part taken in its foundation by workers such as Panum, Gaule, Roser, etc…”44 However, it seems that none of these workers seized upon this concept and appreciated its importance to the degree that Metchnikoff had; certainly, none had further developed and tested what in retrospect was the correct interpretation of the defensive function of phagocytosis.
Following the key experiment in 1882, described previously, Metchnikoff performed many others to test the “phagocytosis theory,” such as the observation of infection in water fleas, which he viewed as a Darwinian struggle between pathogen and host45:
Once they have insinuated themselves into the organism’s inmost part, the spores cause an accumulation of the mobile cells round them, which correspond to the white corpuscles in human blood. A battle takes place between the two elements.
Drawing the analogy with host defense in higher organisms, Metchnikoff described the killing of the spores by “mobile cells,” thus ensuring immunity for the organism. His view of phagocytosis expanded to encompass not only host defense but also organismal development, which he viewed in a teleologic context; he cited the dissolution of the tadpole’s tail by the “pervasion of phagocytes.”46 By the time he moved to Paris to become Chef de Service at the Pasteur Institute in
1888, Metchnikoff had already formulated and tested what had become, according to his view, a cornerstone of the science of immune system. Perhaps what he had not appreciated at the time was that his move to Paris would come to signify to the Germans a complicit alliance with the French. He thus had unwittingly entered a battle that not only had been fought in the political arena but in the laboratory as well.
1888, Metchnikoff had already formulated and tested what had become, according to his view, a cornerstone of the science of immune system. Perhaps what he had not appreciated at the time was that his move to Paris would come to signify to the Germans a complicit alliance with the French. He thus had unwittingly entered a battle that not only had been fought in the political arena but in the laboratory as well.
The Ascendance of Humoral Immunity
Several related developments in the new field of immunology occurred at the end of the 19th century that would seal the fate of cellular immunology for at least 50 years: The discovery by Roux and Yersin47 that toxins alone derived from diphtheria bacilli could reproduce the symptoms of diphtheria; the discovery by von Behring and Kitasato in 189048 of humoral immunity to diphtheria and tetanus and the passive transfer of immunity to diphtheria in animals by von Behring and Wernicke in 189249; and the discovery of alexins (Greek for “without a name”) by Hans Buchner in 189950 and Jules Bordet at about the same time.51 Alexin was renamed “complement” by Ehrlich, as it “complemented” the activity of antibodies. Indeed, the ability of humoral components alone to lyse bacteria (the Pfeiffer phenomenon) or erythrocytes in the absence of phagocytosis51 provided strong independent evidence supporting the humoralists’ claims. The discovery of complement also had practical uses, as complement fixation became the basis of a widely used serologic test for the diagnosis of syphilis, the so-called Wasserman test.52 The collective discoveries of the “humoralists” would lead to the successful treatment of a number of previously intractable diseases, such as diphtheria. Indeed, the first Nobel Prize in physiology of medicine was awarded to von Behring in 1901, “For his work on serum therapy, especially its application against diphtheria, by which he has opened a new road in the domain of medical science and thereby placed in the hands of the physician a victorious weapon against illness and death.” Bordet himself would later be awarded the Nobel Prize “for his studies in regard to immunity.” Among Bordet’s contributions was the development of the complement fixation test together with his brother-in-law, Octave Gengou. This formed the basis of what Bordet termed “serodiagnosis.”
Although the lines in the sand had already been drawn by the mostly Prussian humoralists led by Ehrlich on the one hand and the cellularists led by Metchnikoff on the other, it was Metchnikoff who wrote a letter to von Behring, proposing a scientific “truce”53:
I now believe … we can calmly work side by side. We can mutually support one another, just like the phagocytes and antitoxins, since … the phagocytes receive considerable assistance from the antitoxic property, just as the phagocytes … render great assistance to the organism or respectively its antitoxic powers, since they capture and destroy … bacteria.
In fact, von Behring did not seem rigidly against the cellular school, and Metchnikoff viewed him as supporting the view that “active immunity requires some type of cellular basis.”53,54 It is difficult to know what to make of this passage; it sounds vague and seems to state the obvious, yet it does suggest a degree of flexibility that more intransigent proponents of the humoralist camp seemed to lack at the time. Regardless, scientific reconciliation would not be forthcoming until many years later, although phagocytosis theory was granted a temporary reprieve by the British physician Almoth Wright, who demonstrated the phenomenon of opsonization of bacteria. Wright was the first to describe a mechanism by which humoral and cellular components of immunity cooperate to kill bacteria.55 Wright is possibly best known in his incarnation as Sir Colenso Ridgeon in Shaw’s “The Doctor’s Dilemma.” Ridgeon defined “opsonin” as “…what you butter the disease germs with to make your white blood corpuscles eat them.”56 In what was viewed as a well-deserved but partly symbolic gesture, nevertheless, Metchnikoff and Ehrlich, two exemplars of the opposing schools of immunity, were awarded the Nobel Prize in 1908 “in recognition for their work on immunity.” It would not be until 40 years later that another cell type of the immune system would be first identified as being the source of antibody57 and 70 years later when dendritic cells (DCs) would be first identified as being the key phagocytic leukocyte responsible for initiating the immune response.58
Paul Ehrlich: The Cellularist’s Humoralist
Paul Ehrlich embodies a pivotal figure in the history of immunology. Although he would make many practical discoveries in his long research career, his greatest contribution to immunology, like Jerne’s over a half century later, was a conceptual breakthrough that served to stimulate the field of immunology for years to come. Although I am tempted to consider his “side chain theory” of antibody formation a paradigm shift, that would presume that there was a preexisting paradigm to shift from, when in fact there was no paradigm of antibody specificity to begin with.
Ehrlich began his research career as a medical student. One of his professors was the pathologist Wilhelm von Waldeyer, who introduced the young Ehrlich, who already showed a strong interest in chemistry, to histologic methods for staining cells and tissues. Following further training in several medical schools, he was influenced by the chemist von Bayer and the pathologists Cohnheim, Haidenhain, and Weigert (his cousin). He presented his thesis on histologic staining in Leipzig at the age of 24, in which he was the first to describe mast cells. As noted by Silverstein, the opening sentence of the thesis gave an inkling of his approach to science59:
While in the modern histological literature, directions on tintorial method are already so numerous, and still increase from day to day, yet their theoretical basis has had only a very negligible consideration.
The same year, he was appointed senior physician in the Department of Internal Medicine at the Charité-Hospital in Berlin. The head of the clinic, Friedrich Frerichs, encouraged Ehrlich to continue his histochemical investigations, which led to the identification of neutrophils, eosinophils,
and basophils as well as the diagnosis of his own case of pulmonary tuberculosis.60
and basophils as well as the diagnosis of his own case of pulmonary tuberculosis.60
It is notable that the term “side-chain” (seitenketten in German) was a chemical term in use at Ehrlich’s time meaning much the same as it does today. It is inescapable to conclude that Ehrlich’s focus and interest in chemistry would be the driving force behind his thinking about how antibody is formed, and “selected for,” by antigen. The essence of Ehrlich’s side chain theory, first proposed in 1897 (Fig. 2.2) is well articulated in Ehrlich’s Nobel lecture and is paraphrased here61:
“The relationship between toxin and antitoxin are strictly specific—tetanus antitoxin neutralizes exclusively tetanus toxin, diphtheria serum only diphtheria toxin …”
“For this reason it must be assumed that the (toxin and antitoxin) enter into a chemical bond … fitting each other ‘like lock and key.’”
“The group in the protoplasm, the cell receptor, must be identical with the antitoxin which is contained in solution in the serum of immunized animals.”
“As these receptors, which may be regarded as lateral chains (“seitenketten”) of the protoplasm…become occupied by the toxin, the relevant normal function of this group is eliminated…the deficiency is not merely exactly compensated, but made up to excess (hyperregeneration).”
What Ehrlich proposed purely on theoretical grounds is a brilliant argument based on a combination of inductive and deductive reasoning. Characteristic of Ehrlich, it is lucid, logical, and profound. Beginning with a consideration of a simple chemical bond, Ehrlich somehow ends up with an antibody-producing cell; hence, “the cellularist’s humoralist.”
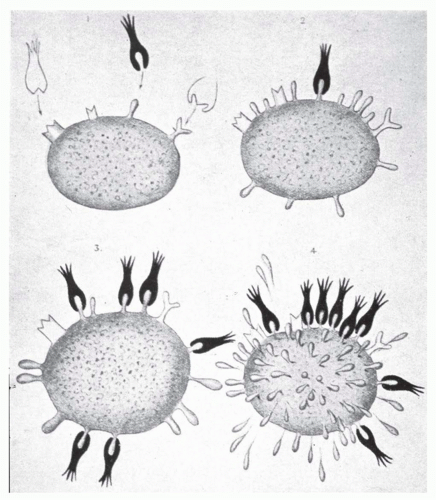 FIG. 2.2. Ehrlich’s “Seitenkette” (Side Chains). From Ehrlich.171 |
Not every biologist was enamored of Ehrlich’s model, and soon after he proposed it, it came under attack, most notably by Jules Bordet. Bordet objected to Ehrlich’s insistence that the specificity of the antigen-antibody reaction required an irreversible bond, whereas Bordet, whose views seemed to be more deductively grounded in his immediate line of investigation (eg, precipitin reactions and complement fixation) thought that adsorptive interactions between antigen and antibody were sufficient to account for specificity.15,60 Although Ehrlich’s side-chain theory provided a logically consistent mechanism to account for antibody specificity, it would later be criticized for failing to account for antibody diversity. That problem would not be conquered for another 60 years in yet another “paradigm shift” when Talmage, Burnet, and Lederberg proposed the clonal selection theory. However, the concept of clonality was not yet conceived of in 1897, and in any case, it is hard to envision how a clonal selection theory could have been developed without the prior description by Ehrlich of antibody selection itself.
IMMUNOLOGY BRANCHES OUT: BENEFICIARIES OF THE EARLY FOCUS ON HUMORAL IMMUNITY
“Man built most nobly when limitations were at their greatest.”
Frank Lloyd Wright
Immunology during the early part of the 20th century was heavily influenced by the early victories of the humoralists. Aside from the spectacular practical implications of the work of von Behring, Bordet, and Ehrlich, among others, the immunologist’s toolkit of the early 20th century immunologist was very limited. Advanced microscopic techniques were not yet available, and cell fractionation techniques had not yet been invented. Given these limitations, it is hard to envision how the gap between antibody and cell could have been bridged any closer than Ehrlich had managed, at least on a theoretical level. However, the focus on humoral immunity did allow for the rapid development of several fields, most notably immunochemistry as well as hematology and allergy. It would be some time before cellular immunology would catch up.
Karl Landsteiner and the Birth of Transfusion Medicine and Autoimmunity
As described by Silverstein, “No single individual contributed as importantly to so many different areas of immunology as did Karl Landsteiner.”15 Landsteiner was born in Baden in 1868 and attended Vienna Medical School. He began his training in internal medicine and studied chemistry with Emil Fischer, who would receive the Nobel Prize in chemistry in 1902. He transferred to the Department of Pathological Anatomy, home to Erdheim, Billroth, Escherich, and other accomplished scientists, where he remained until
1907.62 Landsteiner’s first major accomplishment was the discovery of the human ABO blood group antigens at the turn of the century.63,64 His motivation was succinctly described in his Nobel lecture speech65:
1907.62 Landsteiner’s first major accomplishment was the discovery of the human ABO blood group antigens at the turn of the century.63,64 His motivation was succinctly described in his Nobel lecture speech65:
… proteins in individual animal and plant species differ and are characteristic of each species … The problem raised by the discovery of biochemical specificity peculiar to a species … was to establish whether the differentiation extends beyond the species and whether the individuals within a species show similar though smaller differences.
His experiment was a simple one, in which he applied the sera of six heathy men, including himself, to red blood cells of each, and noted that the sera of the men reacted differently with each other—no serum reacted with that same individual’s red blood cells. At the end of the paper, Landsteiner noted that the results could account for the variable clinical consequences of human blood transfusion —and thus was borne the discovery of the ABO red blood cell antigens that would later become useful in blood typing prior to transfusions. Many years later, Landsteiner would discover the M, N, P isoantigens in 192766 and the Rh system in 194067).
Landsteiner’s next major contribution was the codiscovery with Donath of the first autoimmune disease, paroxysmal cold hemoglobinurea,68 which challenged Ehrlich’s dictum that such a situation could not occur.69
The organism possesses certain contrivances by means of which the immunity reaction … is prevented from acting against the organism’s own elements and so giving rise to autotoxins … so that one might be justified in speaking of a “horror autotoxicus” of the organism.
The nature of the contrivances was thought to be of “the greatest importance” by Ehrlich, who later stated70: “According to our present investigations either the disappearance of receptors or the presence of autoantitoxin is foremost among these contrivances.” Depending on how this statement is interpreted, it could be viewed as Ehrlich’s formulation of either clonal deletion or anti-idiotypes. Two years after the discovery of paroxysmal cold hemoglobinurea, an Italian ophthalmologist, who observed sympathetic ophthalmia, a disease in which damage to one eye leads to inflammation of the opposite eye, speculated that this disease was due to “autocytotoxins.”71 Autoimmunity research was taken up by a few others, but perhaps owing to either a misinterpretation of Ehrlich, or possibly due to deference to his authority in the field, progress was slow. It would not be years later, until the discovery of the role of sensitization of the newly discovered Rh antigen as an etiology of erythroblastosis fetalis,72,73 that autoimmunity became an active area of research for immunologists. The first time that autoimmunity was first associated specifically with arthritis was 1957, when Kunkel and colleagues discovered what came to be called “rheumatoid factor,” large complexes of immunoglobulin (Ig)M directed against IgG in the sera of some patients with rheumatoid arthritis.74 This observation fundamentally changed the field of rheumatology.75
Landsteiner received the Nobel Prize in 1930 “for his discovery of the human blood groups.” Ironically, upon receiving the prize, it is said that he felt the prize should have been awarded for his work on haptens, which would play a great role in the development of the growing field of immunochemistry.
Discovery of Hypersensitivity: The “Other Work”
In 1901 to 1902, Paul Portier and Charles Richet were attempting to raise tolerance in laboratory animals to actinotoxin, an uncharacterized toxin derived from tentacles of sea anemones. Their experiments appeared to be unsuccessful, and, in some cases, it appeared that the animals actually became sensitized to the antigen. They repeated their studies using dogs and obtained completely unanticipated results: All eight dogs collapsed and died within minutes after receiving a relatively small dose. First thinking the results were due to experimental error, they later realized that the sensitized animals had all been exposed to antigen 14 to 23 days previously.76 They proposed the name “aphylaxis” (against protection), a term later changed to “anaphylaxis.”77 Richet continued his investigations on anaphylaxis and was awarded the Nobel Prize in 1913 for his work. Soon after Portier and Richet made their seminal discovery, Maurice Arthus was able to induce a localized form of anaphylaxis (swelling and ultimately gangrene) by repeated subcutaneous injections of horse serum, considered fairly nontoxic.76 Yet, a third type of hypersensitivity was described by the pediatricians von Pirquet and Schick,78 who noted that vaccinated children occasionally developed fever, joint pains, rash, diarrhea, and hypotension. They concluded that the clinical features of what is now called “serum sickness” were not a direct result of the injection of antiserum, but the outcome of “when antigen and antibody meet.” As von Pirquet later explained,79
We are able to observe the effects of the toxic body formed when antigen and antibody meet, that is, the serum disease. We see that at the time when the antibody arises, and therefore the antigen disappears, symptoms of general disease occur. The supposed connection is that these symptoms are due to toxic bodies formed by this digestion of the allergen through the antibody.
Although von Pirquet does not precisely define what he meant by “toxic body,” some have interpreted this to mean antigen-antibody complexes. It is possible that he was reluctant to be more specific as he did not actually have a way of observing or quantifying the complexes; it is also possible that he did not have a ready explanation for how such a complex, if formed, could lead to the symptoms and signs of serum sickness. It would not be until many years later that Frank Dixon and colleagues would precisely delineate the nature of the immune complexes.80 Nevertheless, it is clear that von Pirquet and Schick viewed this phenomenon as
lying along a continuum with the normal immune response, or rather being a necessary component of it. As they later stated,81
lying along a continuum with the normal immune response, or rather being a necessary component of it. As they later stated,81
The conception that the antibodies, which should protect against disease, are also responsible for the disease, sounds at first absurd… One forgets too easily that the disease represents only a stage in the development of immunity, and that the organism often attains the advantage of immunity only by means of disease.
It is von Pirquet and Schick who coined the term “ allergy” (from the Greek allos, other, and ergon, work).79 By highlighting the role of tissue injury in promoting immunity, they closed the loop that Metchnikoff had begun at the end of the 19th century. This theme would be revisited and expanded upon in the latter part of the 20th century when it was discovered that the inflammation that accompanied the innate immune response was a necessary prequel to the acquired immune response.
THE LONG JOURNEY FROM THE DAWN OF IMMUNOCHEMISTRY TO THE STRUCTURE OF IMMUNOGLOBULINS
Challenges to Ehrlich: The Problem with the “Keys”
One of the difficulties that Ehrlich faced was bridging the gap between the conceptual basis of antibody specificity and the actual basis of antibody specificity. If his side-chain theory was correct, then every cell involved in antibody production would be capable of reacting against every possible antigen it might encounter. Even without considering the cellular origins of antibodies, Ehrlich’s critics, such as the Viennese Max von Gruber, raised the question of how the astonishingly large number of different specificities of the antibody molecules themselves could be generated.15 The size of the repertoire seemed impossibly large if every “lock” had a unique “key.” Landsteiner became von Gruber’s assistant at the University of Vienna in 1896, and he inherited von Gruber’s critical view of Ehrlich’s theory. Landsteiner’s early approach to this problem was to adopt Bordet’s “colloid” explanation of the antigen-antibody interaction,15 which rejected covalent interactions in favor of multiple weaker interactions, a theme that was later taken up by Pauling, with some interesting consequences. Later, stimulated by the work by Obermeyer and Pick, who described chemical modifications of proteins,82 Landsteiner used structurally related reactive chemicals to derivatize proteins. He showed that antisera raised against one of the chemically modified proteins would react to varying degrees with proteins modified by structurally similar, but not identical, reactive molecules. These results were interpreted as being incompatible with Ehrlich’s “lock and key” specificity but called for a more nuanced view of antigen-antibody specificity. It became immediately apparent that the size of the repertoire could be greatly enhanced if one allowed for such graded degrees of binding affinities.
Immunoglobulin Structure: The “Keys” to the Problem
It was clear that further progress on defining the physicochemical nature of the antigen-antibody reaction required the development of specific tools that were unavailable at the turn of the century. Antibodies, whose chemical structures were unknown at the time, were considered “colloids” (from Greek kolla, glue), a suspension of particles suspended in a continuous phase of another component. In 1924, the Swedish chemist Svedberg designed a centrifuge based on a modified milk separator. The centrifuge could develop a centrifugal field of up to 5000 g and enabled Svedberg to measure the molecular mass of hemoglobin83; he was the first to determine the molecular mass of macroglobulins, derived from a patient with Waldenström macroglobulinemia, which we now know as IgM. A student in Svendberg’s laboratory, Arne Tiselius, developed gel electrophoresis,84 which allowed for the separation of molecules based on charge and size. These tools enabled Michael Heidelberger to establish the field of “immunochemistry.” Heidelberger devoted nearly his entire research career pursuing the implications of a simple but profound discovery he made with Oswald Avery in 1923 that type-specific antigens of pneumococcus bacteria are complex polysaccharides. Over the next three decades, this discovery enabled him to determine, for the first time, the exact weight and chemical composition of antibodies, antigens, and complement. He showed that antibodies are multivalent proteins and used these insights to devise a simple vaccine against pneumonia whose effectiveness was first proven in soldiers who fought in World War II.85
The Chemical Nature of Immunoglobulin Molecules
By 1950, it was appreciated that antibodies were proteins of 150,000 molecular mass containing bivalent antigencombining sites. Based on Porter’s observation that Igs could be split into smaller products by enzymes such as papain, yielding Fab fragments that bind antigen and Fc (crystallizable) portions that do not, Edelman and Poulik further defined Ig structure by hypothesizing that Bence-Jones proteins, derived from myeloma cells, were free light chains of Ig molecules.86 As this hypothesis appeared to be correct, this provided a means of obtaining a ready supply of homogeneous Ig subunits. Reduction of disulfide bonds led to still different products, enabling them to propose that the Ig molecule consists of two light chains and two heavy chains, and that the antigen-binding site consisted of contributions from both heavy and light chains.86 Further refinement of techniques in protein chemistry allowed Edelman and Porter to work out the primary structure of Ig molecules, for which they received the Nobel Prize in 1972.
CONFRONTING THE SIZE OF THE REPERTOIRE
The value of a large repertoire from which to select is appreciated by any performing musician. Yet in the 1950s, the repertoire problem was unsolved, leading to competing theories of how a large repertoire of diverse antibodies are generated. There were two mutually exclusive leading schools of thought: “instruction” theories and “selection” theories.
Instruction Theories of Antibody Diversity
As noted by Silverstein,15 the first description of antigen as template was by Bail and Tsuda, who proposed in 1909 that antigen persists after its encounter with antibody, and that by so doing, it leaves an impression on the antibody.87 This concept was further refined by Breinl and Haurowitz, who suggested that antigen is carried to the site of protein formation, where it would serve as a template to instruct antibody formation.88 Finally, in 1940, Linus Pauling provided a chemical explanation for antigen-directed instruction: that “antibodies differ from normal serum globulin only the way the ends of the polypeptide chain is coiled” as a result of their amino acid sequence, and that “they have accessible a very great many configurations with nearly the same stability.” Under the “influence” of antigen, they “assume configurations complementary to surface regions of the antigen,” forming two active ends, and that after “freeing one end and the liberation of the central part of the chain, it folds up to form the central part of the antibody molecule, with two oppositely directed ends able to attach themselves to two antigen molecules.” This interaction would be further stabilized by weak interactions between antigen and antibody.89 Assuming a degree of degeneracy in the initial antigen-antibody interaction, these theories were consistent with the findings of Landsteiner, who showed that antigen-antibody interactions were not absolutely specific, as envisioned by Ehrlich (Fig. 2.3). In retrospect, these theories had great chemical appeal; however, a central weakness is that if the initial interactions between antigen and antibody are degenerate, that implies that the interactions cannot be of high affinity (otherwise they would not be degenerate). Yet, if the initial interactions are weak, how would they occur in the first place? At some level, there has to be a certain degree of preexisting antibody specificity, which implies a preexisting repertoire. Although the instruction theories of antibody selectivity helped explain some of the specificity of the antigen-antibody interactions, they could not account for all of them. It is notable that the underlying basic principles have been since invoked for other encounters in the immune system. For example, a “bar code model” of interactions between the T-cell receptor (TcR) and peptide-major histocompatibility complex (MHC) (pMHC) has recently been proposed, in which the initial encounter between TcR and pMHC is governed by strong interactions, followed by “scanning” the epitope and modest changes in conformation of the TcR, leading to strengthening of the TcR-pMHC association.90
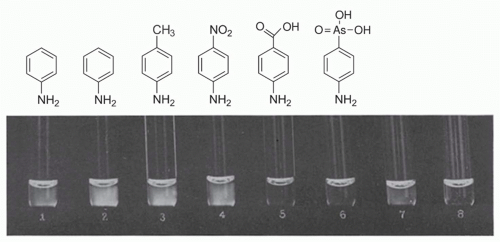 FIG. 2.3. Demonstration of Serologic Specificity by Landsteiner and Scheer. Reactions from immune serum for aniline with various azoproteins and with unchanged horse serum reading after 15 minutes. (1) azoprotein from chicken serum and aniline, (2) azoprotein from horse serum and aniline, (3) azoprotein from horse serum and para-toluidine, (4) azoprotein from horse serum and para-nitroaniline, (5) azoprotein from horse serum and para-aminobenzoic acid, (6) azoprotein from horse serum and para-arsanilic acid, (7) unchanged horse serum, (8) saline control. Modified from Landsteiner and van der Scheer.486 |
Jerne’s Natural Selection Theory of Antibody Formation
Instruction theories of antibody specificity persisted through the 1950s, when they were modified to include participation of enzymes to act as intermediaries between antigen and antibody as well as the newly discovered structure of deoxyribonucleic acid (DNA). In 1955, Niels Jerne published a highly influential paper in which he proposed a new theory of antibody formation that he described as the “natural selection theory of antibody formation.”91
The antigen is solely a selective carrier of spontaneously circulating antibody to a system of cells which can reproduce this antibody. Globulin molecules are continuously being synthesized in an enormous variety of different configurations…among which… will be fractions possessing affinity toward any antigen to which the animal can respond.
Jerne referred to these preexisting antibodies “natural antibodies,” and went on to state that antigens selectively attach to those globulin molecules that happen to have a complementary configuration. According to Jerne, once the interaction occurs, the antigen-antibody complexes may be engulfed by a “phagocytic cell,” at which point the antigen is eliminated. The antibody within the phagocytic cell can remain or be transferred to another cell, which is the signal for reproduction of the same specific antibodies. More antibody is released into the circulation, resulting in a larger percentage of specific circulating antibody. Jerne states that “the reproduction need not be highly faithful; copying mistakes will be harmless and may occasionally produce an improved fit.”91 These are remarkable concepts, as Jerne appeared to have invoked Metchnikoff, a Darwinian interpretation of antibody selectivity at the organismic level, as well
as to have anticipated affinity maturation. It is easy to see why Jerne’s ideas were so influential. However, the central problem, the lack of an explanation for the huge existing repertoire, which hampered the instruction theories of antibody diversity, as well as Ehrlich’s side-chain theory was still unanswered. Jerne admitted this weakness and speculated that the “spontaneous production of globulin molecules of a great variety of random specificities” may reside in a “… specialized lymphoid tissue, such as that of the thymus.”91 Perhaps a more existential problem was that the flow of information was from the existing preformed antibody to more antibody without any genetic intermediary. What was lacking was a specific mechanism to transfer information between a specific antibody and the specific synthesis of that same antibody. Jerne admitted to this problem and nominated ribonucleic acid (RNA) as a key template; he then stated “ … a protein molecule may determine the order of the nucleotides in the synthesis of RNA,” citing a paper that does not actually make this assertion.91
as to have anticipated affinity maturation. It is easy to see why Jerne’s ideas were so influential. However, the central problem, the lack of an explanation for the huge existing repertoire, which hampered the instruction theories of antibody diversity, as well as Ehrlich’s side-chain theory was still unanswered. Jerne admitted this weakness and speculated that the “spontaneous production of globulin molecules of a great variety of random specificities” may reside in a “… specialized lymphoid tissue, such as that of the thymus.”91 Perhaps a more existential problem was that the flow of information was from the existing preformed antibody to more antibody without any genetic intermediary. What was lacking was a specific mechanism to transfer information between a specific antibody and the specific synthesis of that same antibody. Jerne admitted to this problem and nominated ribonucleic acid (RNA) as a key template; he then stated “ … a protein molecule may determine the order of the nucleotides in the synthesis of RNA,” citing a paper that does not actually make this assertion.91
What accounted for this conceptual block that had persisted for more than half a century? On one level, it was ignorance of the “fundamental dogma of molecular biology,” which had not yet been articulated.92 But on another level, it perhaps can be traced back to the decisive victory of the humoralists over the cellularists. So long as the focus was on the antigen-antibody interaction, there was no way to invoke a biologically plausible mechanism of generating a preexisting Ig repertoire and of selectively expanding a specific portion of that repertoire.
Development of the Clonal Selection Theory
In 1957, the American immunologist David Talmage published a review whose focus was allergy; however, in the review, Talmage drew an analogy between natural selection, in which there is “selective multiplication of a few species out of a diverse population” and antibody production. In a very succinct but remarkably insightful passage, he lays out the essence of the clonal selection theory93:
As a working hypothesis it is tempting to consider that one of the multiplying units in the antibody response is the cell itself. According to this hypothesis, only those cells are selected for multiplication whose synthesized product has affinity for the antigen injected.
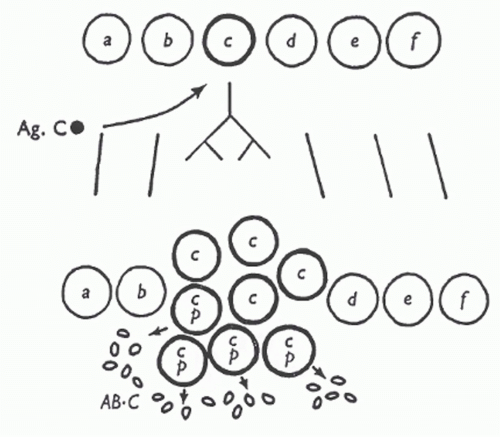 FIG. 2.4. In this diagram, antigen C (Ag.C) is recognized by clone “c,” triggering this clone, but not others, to proliferate. Antibody against Ag.C (AB.C) is indicated at the bottom. From Burnet.246 Copyright ©1959 Sir MacFarlane Burnet. Reprinted with the permission of Cambridge University Press. |
At about the same time, Burnet published his own paper outlining the key aspects of the clonal selection theory94 (Fig. 2.4). In a later interview, Talmage discussed how he provided a preprint of his review to Burnet before he published his landmark paper on the clonal selection theory, but both he and Nossal stated that Burnet developed the theory independently. Talmage thought that the two papers were similar in substance, but that “Burnet had the good fortune to make an analogy of the idea to the clones that grow in bacteria and he gave it a very catchy name, ‘clonal selection.’”95 Among the ideas that led both immunologists to propose the clonal selection theory was the known logarithmic rise in antibody titer during the primary immune response “as if it is a product of some multiplying substance.”95 Other experiments demonstrated that lymphoid cells obtained from previously immunized rabbits that were then transferred to x-radiated recipients were sufficient to induce a recall response in the recipients.96
Although we now know that the clonal selection theory is correct, it took more than 10 years for clonal selection to be widely accepted, according to Talmage.95 Among the key pieces of evidence to prove the theory was the demonstration of antibody production from single cells, by Nossal and Lederberg,97 and the later finding that cognate antigen was capable of aggregating all surface Ig on individual cells,98
which corroborated Nossal and Lederberg’s results. Finally, in 1975, Köhler and Milstein demonstrated the production of monoclonal antibodies from single clones of immortalized plasma cells.99 Along with Jerne, they shared the Nobel Prize in 1984.
which corroborated Nossal and Lederberg’s results. Finally, in 1975, Köhler and Milstein demonstrated the production of monoclonal antibodies from single clones of immortalized plasma cells.99 Along with Jerne, they shared the Nobel Prize in 1984.
The Structural Basis of Antibody Diversity: The Dialectic Revisited
By the mid-1970s, the puzzle of antibody diversity was far from solved—many of the pieces were still missing. The conceptual basis for the clonal selection theory was laid in 1957 by redirecting the focus of investigation from immunochemistry to cell biology. However, what was needed to actually prove the theory was a delineation of the molecular basis for the enormous size of the repertoire. Two lines of investigation converged to provide this evidence: the sequencing of Ig proteins followed by the sequencing of Ig genes. In 1965, Hischmann and Craig sequenced two Bence-Jones proteins and found that there was conservation of the amino acid sequence at the C-termini but considerable diversity at the N-termini.100 In the ensuing years, sequence data on a number of myeloma proteins became available, and in 1970, Kabat and Wu applied statistical criteria to analyze sequencing data from 77 Ig chains. They identified three regions within the 107 residues comprising the light chain variable region that demonstrated a still further degree of variability (ie, hypervariability). They hypothesized that these regions of extreme diversity represented the complementarity-determining residues and suggested by analogy with prokaryotes that they arose through episomal incorporation into the light chain locus by a recombination event.101 If this, indeed, was the underlying explanation for antibody diversity, then there would have to be a very large number of episomal elements to account for a diverse repertoire. This was a different view than the one taken by Dreyer and Bennet 5 years earlier, who proposed that variable region genes that had undergone duplication and spontaneous mutation throughout evolution underwent a “genetic scrambling” event, resulting in their juxtaposition to the constant regions of the Ig genes. They even suggested the involvement of enzymes involved in DNA repair as contributing to such an event.102 Thus, two opposing viewpoints began to emerge to account for the generation of diversity (or GOD, as playfully described by Richard Gershon): somatic mutation of a few genes, as suggested originally by Burnet,94 and supported by Weigert and Cohn’s sequencing data of the mouse λ light chain locus,103 or somatic recombination among many duplicated genes within the germline, as proposed by Dreyer and Bennet102 and later refined by Edelman and Gally104 and Hood and Talmage.105 In the years that followed, various teleologic arguments were proposed to support one theory over the other. In 1976, at least a partial resolution was provided by Hozumi and Tonegawa, who provided evidence that the Vκ and Cκ loci from embryonic DNA rearrange to form a contiguous polypeptide in mature lymphocytes.106 The advent of molecular cloning led to direct proof that the variable and constant regions of the light chain gene had undergone rearrangement at the DNA level.107,108 This was followed by the demonstration by Weigert et al.109 that the Ig Vκ region in the mouse is encoded by multiple V, J, and C regions joined at the DNA level during differentiation of individual lymphocytes. Finally, in 1980, Early et al.110 demonstrated how V, D, and J regions of the Ig locus could recombine to generate a virtually unlimited combination of antibody specificities.
In the ensuing years, the molecular mechanisms governing VDJ recombination were uncovered. These involved recognition of conserved sequences flanking germline V, D, and J segments, introduction of double-strand breaks, potential loss or gain of nucleotides at the coding junctions, and polymerization and ligation to complete the joining process. Many talented scientists contributed to these discoveries, including Alt, Yancopoulos, Blackwell, and Gellert.111 This culminated in the isolation of the recombinase activating genes (RAG) by Baltimore’s group.112, 113 The dominant view that emerged from these studies largely favored the “germline-ists,” reinforced by Tonegawa’s receiving the Nobel Prize in 1984. However, in yet another example of a synthesis of two apparently contradictory approximations of the truth, evidence for somatic hypermutation began to emerge.114,115 Its importance was established when it was causally linked to the generation of B cells with very high affinity antibodies,116 a phenomenon termed “affinity maturation.”117
SPECIALIZATION WITHIN THE IMMUNE SYSTEM
Division of Labor: Discovery of T and B Cells
The first person to demonstrate delayed type hypersensitivity may have been Robert Koch in 1882. On his quixotic pursuit of developing a vaccination against tuberculosis, he injected himself with spent medium from cultures of human tubercle bacilli and noted a particularly severe reaction, including systemic effects.118 Although he was not successful in developing a vaccine against tuberculosis, he recognized the diagnostic potential of this procedure. It was not until 1942, and then later in 1945, that Landsteiner and Chase demonstrated that cells from guinea pigs previously immunized with Mycobacterium tuberculosis or hapten would transfer reactivity to a naïve recipient when challenged with the immunogen.119,120 This was the first demonstration that cells, rather than antibody, transmitted specific immunity, a finding that in some ways vindicated Metchnikoff’s cellular focus.
The identity of the cells mediating the transferred hypersensitivity was unknown. Based on experiments performed decades earlier, as Silverstein has noted,121 James Murphy at the Rockefeller Institute argued that lymphocytes were important in the host resistance to tuberculous infection. Murphy used mice exposed to x-rays or splenectomized to manipulate lymphocyte numbers and showed that conditions that would have been predicted to deplete lymphocytes resulted in early death of the mice due to disseminated tuberculosis.122 In earlier papers, Murphy also showed more directly that lymphocytes were important in graft rejection in transplanted chick embryos. Why were these seemingly important observations ignored? Was it because the
experiments relied to a certain extent on inference, rather than direct proof that lymphocytes were key mediators of tuberculous immunity? Most likely, it was a combination of events: The lingering vestiges of the confrontation between the cellularists and the humoralists and the fact that there was little conceptual basis for understanding how a small innocuous-appearing cell type could participate in immunity.
experiments relied to a certain extent on inference, rather than direct proof that lymphocytes were key mediators of tuberculous immunity? Most likely, it was a combination of events: The lingering vestiges of the confrontation between the cellularists and the humoralists and the fact that there was little conceptual basis for understanding how a small innocuous-appearing cell type could participate in immunity.
In a completely independent line of investigation, it was known for some time that certain strains of mice had a high spontaneous rate of developing lymphocytic leukemia. In attempting to explain the finding that thymectomy of 2-month-old mice failed to develop leukemia, Jacques Miller found that neonatally thymectomized mice appeared ill and revealed a marked deficiency of lymphocytes in blood and lymphoid tissues. Furthermore, these mice failed to reject allografts or xenografts.123 However, not all areas within lymphoid organs were depleted of lymphocytes, consistent with “thymic-dependent” (paracortical areas of the lymph nodes and periarteriolar sheaths of the spleen) and “thymic-independent” regions (follicles and medullary cords). Miller concluded that the thymus was important for the development of a subset of lymphocytes important in allograft rejection.
The involvement of lymphoid cells in antibody production was considered likely in the 1940s, mainly due to “guilt by association.” For example, Erich and Harris injected antigens, such as typhoid vaccine and sheep erythrocytes, into the feet of rabbits, and then compared the formation of antibody to histologic changes in the draining lymph nodes.124 Similar experiments were performed using intravenous injection of antigen, and the appearance of antibody and plasma cells in the spleen appeared to be correlated.125 However, actual proof for the involvement of B cells in antibody production was quite accidental.126 In 1952, Bruce Glick, a young doctorate student at Ohio State University, was investigating the function of an obscure avian organ, the “bursa of Fabricius.” He removed the organ, which did not result in a discernable phenotype. A fellow graduate student asked to use one of Glick’s birds to develop an antibody against Salmonella and found that the bursectomized chicken failed to make antibodies. This led to the publication that eventually appeared in Poultry Science describing the role of the organ in the generation of bursa-derived or “B” cells.127 As there was no anatomic equivalent of the bursa in mammals, an exhaustive search finally revealed the bone marrow origin of these cells. It was also found that thymusderived cells, later named “T cells,” were needed to “help” B cells produce antibody.128,129,130 These distinctions were further clarified when Cooper et al.131,132 showed that T cells were required for delayed-type hypersensitivity and graft versus host reaction. Thus was borne one of many central dichotomies that Mazumdar133 has argued drives the field of immunology.
In 1957, Gowans134 cannulated the thoracic duct of rats and measured the rate of flow of the lymph. He suggested that “the continuous entry of living lymphocytes into the blood may be essential for maintaining the output of lymphocytes from the thoracic duct.” He later showed that intravenous transfusion of radiolabeled lymphocytes resulted in appearance of the radiolabeled cells into the thoracic duct, thus defining the continuous recirculation of lymphocytes from the lymphatics to the blood.135 In retrospect, these experiments were critically important in understanding how lymphocytes are constantly patrolling the lymphatics, vigilantly on the lookout for antigen.
TRANSPLANTATION BIOLOGY AND THE PURSUIT OF IMMUNOLOGIC TOLERANCE
The history of transplantation began many hundreds of years ago and was vigorously pursued by surgeons and tumor researchers during the early part of the 20th century. These events are well summarized in several texts, notably Brent’s A History of Transplantation Immunology and Silverstein’s A History of Immunology. The influence of these early workers, particularly Carrel’s, on the surgical aspects of transplantation is clear, but their impact on the field of transplantation immunology was limited because the conceptual framework of immunology was still rudimentary. Analogous to the role that smallpox had in catalyzing early vaccine development, large-scale bombing campaigns in World War II resulted in many civilian and military burn victims who required skin grafting, compelling surgeons to develop better techniques to avoid homograft rejection. The British zoologist Peter Medawar was assigned to the War Wounds Committee of the Medical Research Council. In 1943, Medawar and Gibson136 published “The fate of skin homografts in man” based on a single burn victim who received multiple “pinch grafts” of skin. The authors concluded that autografts succeed, whereas allografts fail after an initial take, and that the destruction of the foreign epidermis is brought about by a mechanism of active immunization. Medawar returned to Oxford University to study homograft rejection in laboratory animals and proved that this was an immunologic phenomenon. Medawar137 concluded that the mechanism by which foreign skin is eliminated belongs to the category of “actively acquired immune reactions.” Shortly after this publication, Ray Owen138 published the provocative finding that dizygotic twin calves, who share a common circulatory system in utero, exhibit chimaerism with respect to their twin’s erythrocytes and fail to produce antibodies against each other’s erythrocytes. This led Burnet and Fenner139 to predict that introduction of a foreign antigen early enough in life would fail to elicit an immune response. Medawar reasoned that the successful exchange of skin grafts between dizygotic calves would verify this hypothesis. Together with his postdoctoral fellow Rupert Billingham, he performed a series of grafting experiments that provided direct support for this model.140
Mammals and birds never develop, or develop to only a limited degree, the power to react immunologically against foreign homologous tissue cells to which they have been exposed sufficiently early in fœtal life … this phenomenon is the exact inverse of “actively acquired immunity”, and we therefore propose to describe it as “actively acquired tolerance.”
At the same time, Milan Hašek141 in Prague demonstrated successful parabiosis of chick embryos derived from two
different strains. Hašek’s stated motivation to perform the experiment was to determine whether exchange of blood in the different chick strains induced a “mutual metabolic assimilation between parabionts,” rather than to induce a state of immune tolerance. It has been suggested that Hašek’s real motivation for the experiment was to advance the Lysenkoist genetic doctrine to appease the local communist regime in Czechoslovakia.142 Regardless, the result has been reinterpreted as an example of the induction of immunologic tolerance. In 1960, Medawar shared the Nobel Prize with Burnet “for the discovery of acquired immunologic tolerance.”
different strains. Hašek’s stated motivation to perform the experiment was to determine whether exchange of blood in the different chick strains induced a “mutual metabolic assimilation between parabionts,” rather than to induce a state of immune tolerance. It has been suggested that Hašek’s real motivation for the experiment was to advance the Lysenkoist genetic doctrine to appease the local communist regime in Czechoslovakia.142 Regardless, the result has been reinterpreted as an example of the induction of immunologic tolerance. In 1960, Medawar shared the Nobel Prize with Burnet “for the discovery of acquired immunologic tolerance.”
Mechanism versus Metaphor: “Self/Nonself” Discrimination
Medawar’s experiments and Burnet’s formulation of the clonal selection theory occurred at a critical juncture in the history of the evolving field of immunology. Had their development been dyssynchronous, it is doubtful that much progress would have been made on either front. Indeed, transplantation experiments had been performed earlier in the century with little insight as to why allografts failed. The viewpoint that Burnet espoused, that the function of the immune system is to distinguish “self” from “nonself,” has proved to have enormous heuristic value ever since its formulation more than 60 years ago. Is this distinction mostly metaphorical, as suggested by Tauber,143 or does it reflect a more concrete generative reality? To begin to address this question, it is necessary to briefly review a parallel development in immunology, the discovery of the components of the immune system that define molecular self-hood.
Looking Under the Hood: The Discovery of the Major Histocompatibility Complex
The clonal selection theory represented a conceptual breakthrough in the history of immunology, but it did not explain how lymphocytes actually recognize antigen. These insights would eventually come from three sources over the span of 20 years: studies of the genetics of graft rejection in inbred strains of mice by George Snell in the 1940s, studies of the agglutination of white blood cells by sera from transfused patients by Jean Dausset in the 1950s, and studies of the immune response to simple antigens in guinea pigs by Baruj Benacerraf in the 1960s. Snell was interested in identifying genes that controlled the ability of mice to resist tumor transplants. He pioneered the use of congenic mice, which are genetically identical except for a single region or locus. Snell observed that tumor grafts were accepted between inbred mice but not between mice of different strains. The same was true for normal tissues. Snell termed the underlying genes “histocompatibility” genes. In collaboration with Peter Gorer, who had prepared antisera that reacted with cells from one mouse strain but not another, Snell established that the major histocompatibility locus corresponded to a reactivity that Gorer had designated antigen II, renamed locus histocompatibility 2 or H-2.144 Two loci within this region, designated K and D, carried genes specifying antigens involved in triggering graft rejection. In the 1950s, Dausset observed that patients who had received many blood transfusions produced antibodies that could agglutinate white blood cells from donors but not the patient’s own cells. Several of the patients produced antibodies against the same antigen.145 Subsequent family studies indicated that a genetically determined system, named “human leukocyte antigen” (HLA) system, was found to be the ortholog of H-2 in the mouse. Dissection of the human system would take many years, during which time transplantation surgeons made use of the emerging findings to assist in tissue typing. In the 1960s, Baruj Benacerraf, an immunologist working at New York University, noticed that some outbred guinea pigs responded to simple antigens by developing delayed hypersensitivity reactions upon challenge while others did not, and that this was under genetic control. He termed these genes “immune response genes.” McDevitt and Sela observed similar genes in mice, and McDevitt showed that they are encoded in the MHC.146 In 1980, Benacerraf, Snell, and Dausset shared the Nobel Prize “for their work on genetically determined structures of the cell surface that regulated immunologic reactions.”
Of course, the identification of the HLA region and the subsequent cloning of the genes encoded in this region proved to be landmarks in the history of immunology. Besides providing a molecular identity to the key orchestrators of antigen presentation to T cells, the identification of specific MHC alleles with autoimmune diseases has led to important insights into their pathogenesis. Ironically, the first recognized HLA association with human disease, HLA-B27, which was associated with the disease ankylosing spondylitis,144,147 has generated perhaps more heat than light, as there is no definitive mechanism that explains how HLA-B27 is linked to disease pathogenesis, underscoring that the HLA locus, which took so many years to uncover at the genetic level, still has much to teach us.
The Discovery of Major Histocompatibility Complex Restriction as the Molecular Basis for “Self/Nonself” Discrimination
It was known for several years that cooperation between T and B cells occurred in syngeneic or H-2-compatible animals.148,149,150 In 1972, Kindred and Shreffler151 showed that even in nude mice, cooperation between T and B cells required H-2 compatibility; however, the exact role of that H-2 molecules played in this process and the nature of the T- and B-cell interactions remained a mystery. A valuable clue was provided by experiments by Rosenthal and Shevach,152 who demonstrated that efficient presentation of antigen by antigenpulsed macrophages to T cells also required histocompatibility matching. In 1974, Doherty and Zinkernagel sought to understand the role of T cells in the immune response to viral meningitis. They theorized that it was the strength of the immune response that caused the fatal destruction of brain cells infected with this virus. To test this theory, they mixed virusinfected mouse cells with T lymphocytes from other infected mice. The T lymphocytes did destroy the virus-infected cells, but only if the infected cells and the lymphocytes came from a genetically identical strain of mice. T lymphocytes would
ignore virus-infected cells that had been taken from another strain of mice.153 Further experiments strongly suggested that the same TcR that recognizes viral antigen also recognizes the MHC molecule.154 The implications of the Zinkernagel-Doherty experiment were profound. First, it established the principle of MHC restriction: T cells recognize antigen only in the context of MHC molecules. Second, the experiment established that cytotoxic TcR-bearing cells must recognize two separate signals on an infected cell before they can destroy it. One signal is a fragment of the invading virus that the cell displays on its surface and the other is a self-identifying tag from the cell’s MHC molecules; it was felt likely that the same TcR probably recognizes both. Thus, the experiment pointed to the identity of the molecular structure that constituted immunologic “self”—it is the MHC molecule—and therefore a virus-infected cell bearing MHC molecules was likely to constitute “altered” or “nonself.” Finally, the fact that MHC is highly polymorphic implies that any given allelic product will be capable of forming a different altered self from other MHC allelic products; thus, the specific identity of the MHC molecule itself determines the strength of the immune response.
ignore virus-infected cells that had been taken from another strain of mice.153 Further experiments strongly suggested that the same TcR that recognizes viral antigen also recognizes the MHC molecule.154 The implications of the Zinkernagel-Doherty experiment were profound. First, it established the principle of MHC restriction: T cells recognize antigen only in the context of MHC molecules. Second, the experiment established that cytotoxic TcR-bearing cells must recognize two separate signals on an infected cell before they can destroy it. One signal is a fragment of the invading virus that the cell displays on its surface and the other is a self-identifying tag from the cell’s MHC molecules; it was felt likely that the same TcR probably recognizes both. Thus, the experiment pointed to the identity of the molecular structure that constituted immunologic “self”—it is the MHC molecule—and therefore a virus-infected cell bearing MHC molecules was likely to constitute “altered” or “nonself.” Finally, the fact that MHC is highly polymorphic implies that any given allelic product will be capable of forming a different altered self from other MHC allelic products; thus, the specific identity of the MHC molecule itself determines the strength of the immune response.
Although the Zinkernagel-Doherty experiment answered many questions, the steps between encounter of antigen by an antigen-presenting cell (APC) and presentation of that antigen to a T cell was somewhat of a “black box.” It had long been thought that intact antigen was presented to T cells, but it was not until 1981 that Ziegler and Unanue155 showed that an antigen processing event was necessary for I-region (MHC class II)-restricted antigen presentation to T cells. This appeared to require antigen processing in a lysosomal-like compartment.156 Peptide loading is a complicated affair, involving prior binding of MHC class II by an “invariant chain” to prevent premature loading of incompletely folded proteins in the endoplasmic reticulum.157,158 Peptide loading requires proteolysis of the invariant chain.159,160 Mellman and colleagues would go on to show that the actual compartment in which antigen loading occurred is uniquely specialized for antigen presentation in B cells161 and DCs.162 In a landmark paper, Unanue and colleagues purified MHC class II molecules from 1011 B cells and showed a 1:1 binding with peptide and MHC class II I-Ad, but not I-Ak,163 thus demonstrating MHC restriction at the biochemical level.
The processing events required for MHC class I restriction seemed more elusive, as some of the molecular components required for this were not known at the time. It was suspected that an intracellular proteolytic event was needed to process antigen, but that was not proven until 1994 when Rock et al.164 showed that proteasomal inhibitors blocked degradation of most cell proteins and subsequent generation of peptides presented on MHC class I molecules. The actual mechanism by which peptides generated in the cytosol gained entry into the secretory compartment was provided in 1990, when four groups announced the identification of a member of a family of ABC transporters, called “TAP,” which provided this function.165,166,167,168 Finally, in 1987, Bjorkman et al.169,170 demonstrated that the antigen in question was a peptide actually bound to the groove of the MHC class I molecule.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree