Occurrence in pediatric HGG (%)
Occurrence in pediatric AA (%)
Occurrence in pediatric GBM (%)
Occurrence in adult HGG (%)
Reference
Akt overexpression
79 (42/53)
Pollack et al. (2010)
BRAF V600E mutation
33 (3/9)
0 (0/11)
Schiffman et al. (2010)
CDKN2A inactivation
22 (2/9)
27 (3/11)
Schiffman et al. (2010)
19
Paugh et al. (2010)
EGFR amplification
10 (1/10)
11 (2/18)
30–40
Nakamura et al. (2007)
2.9 (1/34)
Pollack et al. (2006a)
0 (0/17)
0 (0/15)
Raffel et al. (1999)
7.4 (2/27)
Bredel et al. (1999)
0 (0/24)
Cheng et al. (1999)
EGFR overexpression
80 (17/22)
30–40
Thorarinsdottir et al. (2008)
58 (23/40)
Liang et al. (2008)
28 (8/28)
Nakamura et al. (2007)
36 (14/38)
Pollack et al (2006a)
25.9 (13/54)
Ganigi et al. (2005)
81 (22/27)
Bredel et al. (1999)
11 (2/19)
Sure et al. (1997)
EGFRvIII expression
2.5 (1/40)
10–67
Liang et al. (2008)
17 (6/35)
Bax et al. (2009)
IDH1 mutation
0 (0/9)
0 (0/11)
Schiffman et al. (2010)
36 (5/14)
18 (6/33)
Setty et al. (2010)
MGMT overexpression
11 (12/109)
Pollack et al. (2006b)
MGMT methylation
40 (4/10)
45
Donson et al. (2007)
PDGFR-α amplification
0 (0/28)
10–15
Nakamura et al. (2007)
12
Paugh et al. (2010)
17
Bax et al. (2010)
PTEN mutation/deletion/alteration
33 (13/39)
20–30
Liang et al. (2008)
11 (2/18)
Nakamura et al. (2007)
28 (7/25)
Pollack et al. (2006a)
Rb mutation/loss of expression
7.4 (4/54)
70–85
Ganigi et al. (2005)
TP53 mutation
30 (3/10)
33 (6/18)
30–35
Nakamura et al. (2007)
33 (3/9)
36 (4/11)
Schiffman et al. (2010)
33 (40/121)
Pollack et al. (2002a)
38 (9/24)
Cheng et al. (1999)
24 (4/17)
20 (3/15)
Raffel et al. (1999)
25 (5/20)
Sure et al. (1997)
p53 overexpression
35 (14/40)
Liang et al. (2008)
35 (41/115)
Pollack et al. (2002a)
53.7 (29/54)
Ganigi et al. (2005)
A strong association between proliferation index and outcome was found using samples derived from the multi-institutional CCG 945 study (Pollack et al. 2002b). MIB-1 labeling was lower for tumors classified as AA compared to GBM. Furthermore, there was a significant inverse correlation between proliferative index and PFS. Five-year progression-free survival (PFS) was 33 ± 7 % in 43 patients whose tumors had MIB-1 indices of less than 18 %, 22 ± 8 % in the 27 patients whose tumors had indices between 18 % and 36 %, and 11 ± 6 % in the 28 patients whose tumors had indices greater than 36 % (p = 0.003) (Pollack et al. 2002a). These results suggest MIB-1 index is a prognostic factor.
Studies have shown that pediatric high-grade gliomas have a high incidence (40.5 %) of mutations in the tumor suppressor gene TP53 (Pollack et al. 2006a). Although p53 mutations have not been linked to prognosis in adults, low p53 expression has been shown to correlate with improved 5-year PFS in children. Those with low p53 expression by immunohistochemistry had a 44 % PFS rate compared to 17 % in patients with p53 overexpression (Pollack et al. 2001, 2002a). Several other mutations and altered expression patterns have been identified in pediatric high-grade gliomas, although for many of them, the prognostic significance remains less well defined. Compared with adults, children with high-grade gliomas have been found to have less frequent amplification of the epidermal growth factor receptor (EGFR) gene and less frequent mutations in the tumor suppressor PTEN (Bredel et al. 1999; Finlay et al. 1995; Nakamura et al. 2007; Pollack et al. 2006a). Despite the low rate of PTEN mutations, however, about 80 % demonstrate activation of the PI3-kinase/Akt/mTOR pathway, potentially through altered PTEN promoter methylation (Pollack et al. 2010; Mueller et al. 2012). Overexpression of Y-box-protein 1 (YB1) has been observed in many pediatric GBMs, a subset of which shows Ras and Akt pathway activation (Faury et al. 2007). Also, high-resolution genomic analysis of de novo pediatric high-grade gliomas revealed the tyrosine kinase receptor gene PDGFRΑ to be a target of focal amplification (Paugh et al. 2010).
In more recent studies, somatic mutations in H3F3A, encoding histone H3.3, have been identified in a third of non-brainstem pediatric glioblastomas (Schwartzentruber et al. 2012; Sturm et al. 2012; Wu et al. 2012). The mutations affect either amino acid K27 or G34, and the two mutations have been found to define different epigenetic subgroups, with distinct global methylation patterns. The K27 cluster has a median age of 10 and includes tumors that are typically midline (thalamus, brainstem, spinal cord), often with TP53 mutations and with a poor prognosis. The G34 cluster has a higher median age of 18, is localized to the cortex, and is associated with improved prognosis.
In addition to the two H3F3A-mutant subgroups for childhood glioblastoma, a third, nonoverlapping subgroup has been defined as having mutations in the isocitrate dehydrogenase (IDH) 1 gene (Sturm et al. 2012). Those tumors showed global hypermethylation and had improved overall survival. Previous analysis of CCG 945 samples had shown IDH1 mutations to be more prevalent in older children, including 7 of 20 tumors from children 14 years or older but 0 of 23 tumors from younger children (p = 0.0024) (Pollack et al. 2011). No mutations in IDH2 were observed in either group. Both 1-year event-free survival and overall survival were significantly better for the older children with IDH1 mutations.
Other chromatin regulators are mutated in high-grade gliomas in children. The α-thalassaemia/mental retardation syndrome X-linked (ATRX) and death-domain-associated protein (DAXX) genes were found to be mutated in 31 % of pediatric GBMs (Schwartzentruber et al. 2012). The genes encode two proteins in a chromatin-remodeling complex that is required for H3.3 histone incorporation at pericentric heterochromatin and telomeres. Furthermore, the mutations are associated with a telomerase-independent mechanism of lengthening their telomeres.
Mutations in the serine/threonine protein kinase B-Raf have been found in many cancers, and a genome-wide search detected BRAF mutations in several malignant astrocytoma samples in children (Schiffman et al. 2010). On further analysis, about 10 % of malignant astrocytomas have been found to harbor BRAFV600E mutations (Nicolaides et al. 2011), although the mutation is rare in adult gliomas. Individual case reports have demonstrated encouraging results with targeted agents in BRAF-mutant pediatric glioblastoma (Robinson et al. 2014), and clinical trials are in progress.
Current studies aim to further delineate the underlying molecular biology of high-grade gliomas in children. Given the rarity of these tumors, however, significant sample sizes can only be obtained through multi-institutional studies.
2.4 Clinical Features
Exact signs and symptoms caused by high-grade supratentorial gliomas depend upon the anatomic location, biologic aggressiveness, and patient age. These signs and symptoms may be nonspecific, such as those resulting from the effects of increased intracranial pressure, or they may be directly related to the location of the tumor. Nonspecific symptoms include headache, nausea, and vomiting. Focal symptoms may include hemiparesis, monoparesis, hemisensory loss, dysphasia, aphasia, and impairment of recent memory.
Worrisome features of headaches that should alert the clinician are those that wake the child up from sleep, occur on awakening in the morning, involve nausea and vomiting, cause consistent focal pain, worsen with Valsalva maneuvers, progress in severity, fail to respond to any therapy, or occur in the setting of an abnormal neurologic exam (Duffner 2007). In infants with open cranial sutures, tumors may reach a massive size with gradual increase in head circumference without signs of increased intracranial pressure. Subtle symptoms such as increased irritability, change in feeding pattern, and failure to thrive are often misinterpreted.
The time from first symptom to diagnosis is shorter in high-grade gliomas than in low-grade tumors (Mehta et al. 2002; Duffner 2007). Malignant gliomas are less frequently associated with seizures and are more likely to cause focal neurologic deficits, mainly due to infiltration of normal tissue or local mass effect. Disseminated disease at presentation is rare, in contrast to cases involving other malignant pediatric brain tumors such as supratentorial primitive neuroectodermal tumors and medulloblastomas (Benesch et al. 2005).
2.5 Diagnostic Imaging
Magnetic resonance imaging (MRI) and computerized tomography (CT) are essential tools in the diagnosis and treatment of brain tumors. Although CT is more commonly available and can be performed quickly in children, MRI provides higher sensitivity in differentiating tumor tissue from normal brain, allowing for more detailed anatomic characterization of the lesion. MRI should therefore be obtained in all children with a suspected brain tumor. A complete series should include the following sequences: T1-weighted axial and coronal (both before and after gadolinium), T2-weighted axial and coronal, and fluid-attenuated inversion recovery (FLAIR). In addition, sagittal plane sequences are helpful in defining the anatomy of suprasellar and midline tumors. Other sequences such as fat suppression and MR angiography may also be required in specific situations. Contrast-enhanced neuroimaging of the entire neuraxis should be considered if there is a high index of suspicion for the presence of disseminated disease at the time of evaluation. Newer techniques, such as MR spectroscopy, functional MRI, and perfusion measurements offer the potential for obtaining biochemical and functional information noninvasively (see Chap. 13 for more details).
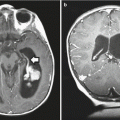
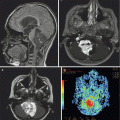
Compared to other malignant brain tumors, high-grade gliomas do not have specific MR imaging features. In general, they appear hypointense on T1-weighted images and hyperintense on T2-weighted sequences. Enhancement occurs after contrast administration with gadolinium, but the degree of enhancement does not correlate with tumor grade (Fig. 2.1). For example, many pilocytic astrocytomas (WHO grade I) demonstrate variable degrees of enhancement. On T1-weighted images, GBMs are typically poorly circumscribed and often demonstrate central areas of low density corresponding to necrosis. This area is surrounded by an area of high density that enhances with contrast, corresponding to actively dividing and proliferating tumor cells. A third, low-attenuation area around the tumor is often seen representing tumor-associated vasogenic edema but also containing infiltrating tumor cells. Peritumoral edema surrounding most high-grade astrocytomas appears as a hyperintense region of signal abnormality on T2-weighted images. The extent of peritumoral edema is underestimated on T1-weighted images. High-grade tumors demonstrate increased blood flow on perfusion studies and elevated choline/N-acetylaspartate (NAA) ratios on MR spectroscopy (see Chap. 13). Thalamic tumors can present as diffuse swelling of the entire thalamus, with or without significant edema (Fig. 2.2).
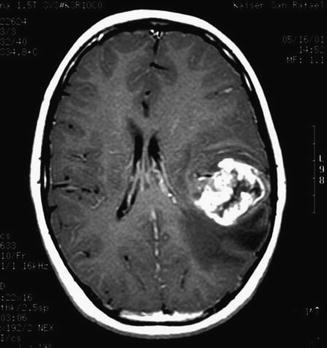
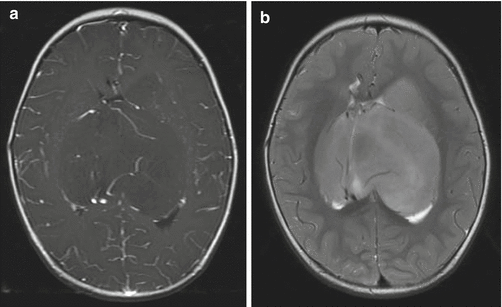

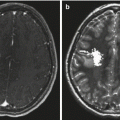
Fig. 2.1
A post-contrast T1-weighted axial MR image of a teenage boy with a glioblastoma who presented with dysphasia. A large tumor in the left frontoparietal area is visible. The margin of the tumor enhances, a central necrotic area is visible, and the low signal region surrounding the mass represents tumor-associated edema

Fig. 2.2
A diffusely infiltrating bilateral thalamic astrocytoma (grade III) in a 2-year-old boy. The tumor does not enhance on T1-weighted images (a). The extent of the tumor is better appreciated on T2-weighted images (b)
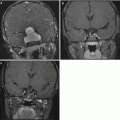
The differential diagnosis based purely on imaging appearance includes other malignant supratentorial hemispheric tumors, such as ependymoma, supratentorial primitive neuroectodermal tumor (PNET), and pleomorphic xanthoastrocytoma. Gliomatosis cerebri is usually diagnosed by widespread infiltration of tumor throughout the hemisphere. A focal lesion is usually not present, although the mass effect can be substantial (Fig. 2.3).
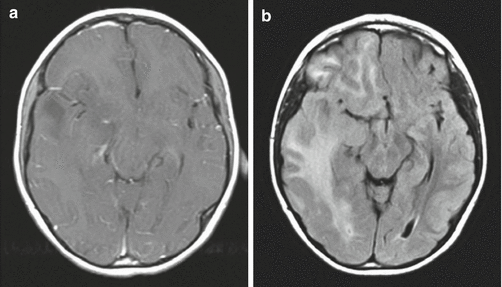

Fig. 2.3
Gliomatosis cerebri. The tumor is difficult to appreciate on T1-weighted images (a). An axial fluid-attenuated inversion recovery (FLAIR) image (b), however, clearly shows a diffuse infiltrative process extending from the frontal lobe into the occipital lobe. Distortion of the cerebral peduncle is seen from the enlarged temporal lobe
Recurrence is an unavoidable feature of most glial neoplasms. For this reason, serial imaging is often the only method to determine whether tumor progression has occurred. Grade II astrocytomas often recur as higher-grade lesions, which usually have imaging features consistent with GBM. Most patients with high-grade gliomas are treated as part of a clinical trial, and the frequency of screening MRIs is generally included in the study design and is frequently performed every 3 months in the first year after diagnosis.
2.6 Treatment
2.6.1 Surgery
Aggressive resection, with preservation of neural function, is the cornerstone of initial management of children with high-grade astrocytomas. The primary objectives are to obtain tissue for pathologic diagnosis, to relieve increased intracranial pressure if present, and to decrease tumor burden. The secondary objective is to perform as extensive a resection as possible with acceptable neurologic outcome. However, high-grade gliomas are diffusely infiltrative lesions, and therefore it remains challenging for the neurosurgeon to define the tumor boundaries during the resection process. For deep lesions or those in eloquent cortex, a stereotactic needle biopsy may be the only surgical option.
Multiple studies have shown gross total resection (GTR) to be linked to longer survival (Finlay et al. 1995; Heideman et al. 1997; Wolff et al. 2002; Yang et al. 2013). In the CCG 945 study, children with high-grade gliomas who underwent GTRs (defined as >90 %) had a 5-year PFS rate of 35 ± 7 % compared to 17 ± 4 % in the group with subtotal resections (STR) (p = 0.006) (Wisoff et al. 1998). This association persisted in subgroup analyses based on histology. Furthermore, the HIT-GBM-C study of multi-agent chemotherapy found 5-year event-free survival to be 13 % overall, but this was improved to 48 % for children with complete resection (Wolff et al. 2010). Complicating the interpretation of data on resection, however, is the inconsistent and subjective methodology used in determining extent of resection.
The feasibility of an open surgical approach depends upon several factors, the most important of which is the exact location of the tumor. Deep lesions within the basal ganglia, thalamus, motor cortex, or brainstem are not amenable to open surgical resection, while tumors in other locations can be accessed through various standard approaches. Thalamic tumors in general are considered unfavorable for resection and therefore carry a dismal prognosis (Fig. 2.2). Other factors that modify the decision to attempt surgical resection are the patient’s clinical condition, age, associated hydrocephalus, and the surgeon’s assessment of risk of neurologic sequelae.
Contemporary neurosurgical methods, including ultrasonography, functional mapping, frameless navigational resection devices, and intraoperative imaging techniques enable more extensive resections with less morbidity. These techniques and intraoperative considerations specific to the pediatric age group are discussed in detail in Chap. 14.
2.6.2 Radiation Therapy
Radiation therapy is the only adjuvant therapy that has been proven to improve survival of children with brain tumors, and it remains the standard of therapy after surgical resection for older children. Children older than 3 years are treated with 50–60 Gy of external beam radiation delivered with standard daily fractions of 1.8–2.0 Gy. Conformal techniques that allow treatment planning based on three-dimensional reconstructions have dramatically advanced the area of radiation oncology (see Chap. 16). Attempts at dose intensification by dose escalation (to a cumulative total dose of 72 Gy) in conjunction with hyperfractionation have failed to improve outcome in the setting of high-grade gliomas (Fulton et al. 1992; Packer et al. 1993). Hyperfractionated radiotherapy utilizes lower doses of radiation per fraction (usually 1–1.1 Gy) administered more than once daily. Decreasing the dose per fraction theoretically spares healthy tissue more than it spares tumor cells, allowing for higher total doses to the tumor, while limiting long-term side effects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree