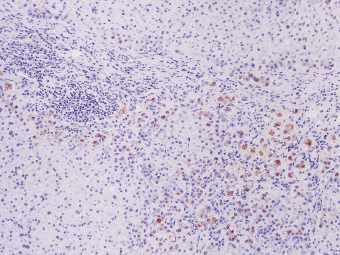
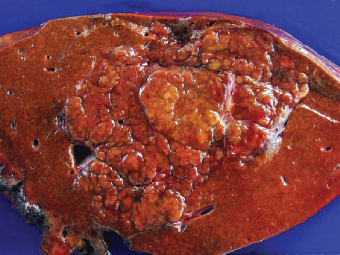
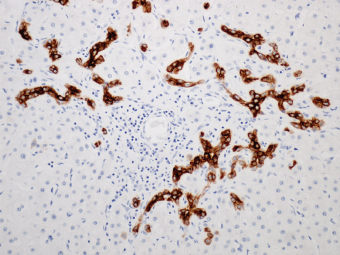
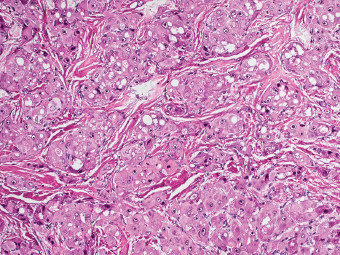
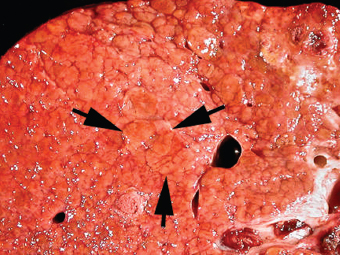
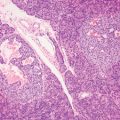
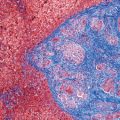
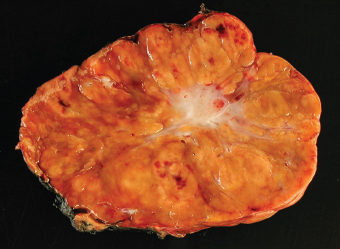
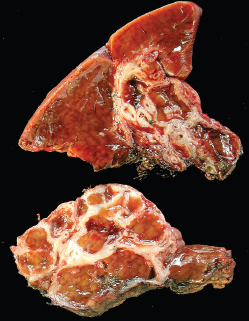
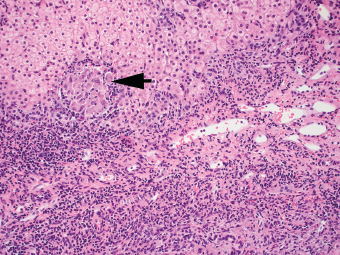
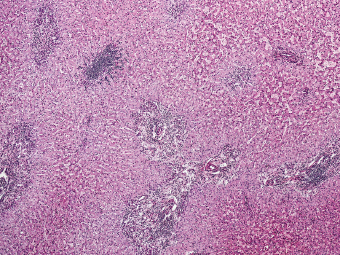
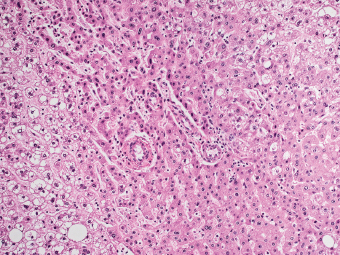
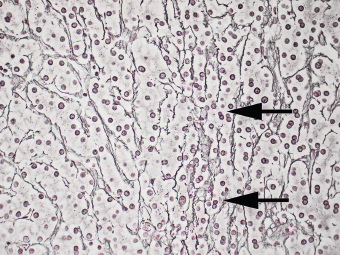
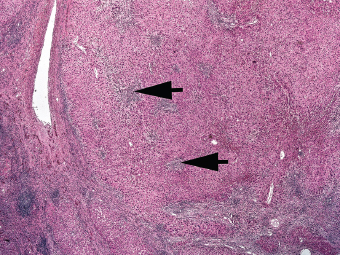
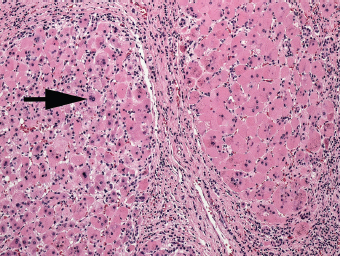
7 Biologic Evolution and Treatment Biologic Evolution and Treatment Neoplastic liver lesions and their mimics are often incidentally discovered during evaluation of the abdomen by imaging studies or in the course of abdominal surgery. Even with the multitude of currently available imaging procedures, many of the neoplastic mimics are considered malignant until resection or biopsy proves otherwise. In the liver, some of the neoplastic mimics are less common than metastatic neoplasms and may have less specific imaging features; hence, knowledge of their existence and histopathologic features is required in narrowing down the differential diagnoses under consideration. Clinical information, such as predisposing condition, associated diseases, and constitutional symptoms, if available, may aid the diagnosis of these lesions. Furthermore, communication and a team approach among surgeons, radiologists, and pathologists are critical in most instances. Fundamentally, neoplasms and neoplastic mimics of the liver arise from the three main compartments of the liver, that is, hepatocyte, bile duct, and mesenchyme; the latter includes stromal and vascular spaces. Based on their predominant features, they can be divided into hepatocellular or hepatoid lesions, ductular or glandular lesions, cystic lesions, spindle cell lesions, fatty lesions, and vascular lesions (Table 7.1). Guided by these predominant features, pathologists can narrow down the diagnosis of neoplasm or neoplastic mimics of the liver under consideration. Many neoplastic mimics are either congenital or developmental in origin. Some of these lesions belong to fibrocystic disease of the liver, consisting of polycystic liver disease, biliary microhamartoma, congenital hepatic fibrosis, Caroli disease, and cholecochal cyst, and they can mimic neoplastic diseases clinically, radiographically, or histologically. Benign hepatocellular or hepatoid neoplastic mimics occur in both cirrhotic and noncirrhotic livers. In the noncirrhotic liver, the most common neoplastic mimic is focal nodular hyperplasia (FNH). It is often found incidentally during abdominal examination. Its distinction from hepatocellular adenoma (HA) is important. In the last several years, there have been many studies confirming HA as monoclonal lesions with specific genetic mutations (401); therefore, HAs are regarded as a hepatocellular neoplasms and complete resection is desirable. In cirrhotic liver, benign hepatocellular lesions include large regenerative nodules, and low and high dysplastic nodules. Large regenerative nodules are considered neoplastic mimics, whereas dysplastic nodules are preneoplastic lesions, supported by an accumulation of evidence in the last two decades that strongly favors the existence of a sequence of events in dysplastic nodules preceding the emergence of hepatocellular carcinoma (HCC) (402). Although dysplastic nodules are not discussed in this chapter, the distinction between dysplastic nodules and large regenerative nodules will be sufficiently elaborated in the differential diagnoses of large regenerative nodules. Another hepatoid neoplastic mimic of HCC is angiomyolipoma (AML), particularly when it is composed of epithelioid cells. In contrast to renal AML, hepatic AML is much less frequently encountered; therefore, it is frequently misdiagnosed due to the lack of familiarity with its wide morphologic spectrum. TABLE 7.1 General Histopathologic Approach to Neoplasm and Neoplastic Mimics of the Liver PREDOMINANT FEATURES NEOPLASTIC MIMICS NEOPLASM Hepatocellular and hepatoid lesions Large regenerative nodule High grade dysplastic nodules, hepatocellular carcinoma Focal nodular hyperplasia Hepatocellular adenoma, fibrolamellar carcinoma Angiomyolipoma, epithelioid type Hepatocellular carcinoma, metastatic sarcoma or melanoma Riedel lobe and accessory lobe Hepatocellular adenoma, carcinoma, metastatic lesion Focal fatty sparing Hepatocellular adenoma, carcinoma, metastatic lesion Liver with submassive or massive necrosis Hepatocellular carcinoma, metastatic lesion Adrenal or pancreatic rest (heterotopic tissue) Hepatocellular adenoma, carcinoma, metastatic lesion Glandular or ductular lesions Biliary hamartoma Metastatic adenocarcinoma, cholangiocellular carcinoma Bile duct adenoma Metastatic adenocarcinoma, cholangiocellular carcinoma Ductular reaction Cholangiocellular carcinoma, metastatic carcinoma Congenital hepatic fibrosis Cholangiocellular carcinoma Cystic lesions Simple biliary cyst Mucinous cystic neoplasm Peribiliary cyst Mucinous cystic neoplasm Ciliated foregut cyst Mucinous cystic neoplasm Caroli disease Metastatic adenocarcinoma Abscess Metastatic neoplasm with necrosis, lymphoma Spindle cell lesions Inflammatory pseudotumor Sarcomatoid hepatocellular carcinoma, embryonal sarcom, metastatic sarcoma Organizing or wall of abscess Sarcomatoid cholangiocellular carcinoma, metastatic sarcoma Hepatic sarcoidosis Metastatic neoplasm, lymphoma Fatty lesions Focal fatty change Hepatocellular adenoma, carcinoma, metastatic lesion Angiomyolipoma, lipomatous type Hepatocellular carcinoma, metastatic lesion Myelolipoma Liposarcoma Pseudolipoma Metastatic adenocarcinoma, liposarcoma Vascular lesions Hemangioma Metastatic neoplasm, angiosarcoma Lymphangioma Metastatic neoplasm, angiosarcoma Peliosis hepatis Metastatic neoplasm, angiosarcoma, infantile hemangioendothelioma Telangiectatic hyperplastic hepatocellular nodule Hepatocellular carcinoma, hepatocellular adenoma and focal nodular hyperplasia FNH is a nodular hepatocellular neoplastic mimic with a central fibrous scar, occurring in a noncirrhotic liver. The formation of FNH is thought to be a hyperplastic response of the hepatic parenchyma to the increase of blood flow secondary to localized vascular malformations, as suggested by Wanless et al. in 1985 (403). This hypothesis is supported by the relatively common coexistence of FNH and cavernous hemangioma, and its relation to other vascular diseases or malformations, such as Budd-Chiari syndrome, hereditary hemorrhagic telangiectasia (Rendu–Osler–Weber disease), or portal vein agenesis (404–407). Recent study has found the alteration in mRNA expression level of angiopoietin genes (ANGPT1 and ANGPT2, with increase in ANGPT1/ANGPT2 ratio), expressed by endothelial cells of dystrophic vessels and sinusoids, which may indicate the involvement of these genes in the pathogenesis of hyperplastic and dystrophic vessels (408). The nonneoplastic nature of FNH is supported by the findings that FNHs are polyclonal lesions and lack the specific mutations found in HAs, such as beta catenin and HNF1-alpha gene mutations (409–411). The distinction between FNH and HA is important as their diagnoses translate to different treatment modalities. FNH is the second most common benign tumor of the liver after hepatic hemangioma. FNH occurs in both men and women, but it is slightly more frequently encountered in women between the ages of 20 and 50 years old (412). FNHs are not associated with oral contraceptive use, although few studies suggested that the use of oral contraceptive may increase the size of FNH (412–414). FNH is commonly a solitary subcapsular lesion in either the right or left lobe. It can be pedunculated and multiple in numbers. When FNHs are found in multiple numbers, a variant of HA referred to as telangiectatic or inflammatory HA should be considered in the differential diagnosis. This lesion exhibits dilated or ectatic blood vessels but lacks a central fibrous scar, and had been confused with FNH because some of the histologic features resemble those of FNH; however, studies have discovered that they are monoclonal lesions and should be classified as a variant of HA (415). The majority of FNHs present as a solitary nodule, while a smaller percentage (approximately 20%) of patients may have multifocal lesions (412). Coexistence with cavernous hemangioma is a common occurrence, where FNH can be found within or immediately adjacent to a cavernous hemangioma. On gross examination, FNH is a well-circumscribed, firm, nodular lesion, and bulging out from the surrounding noncirrhotic liver parenchyma. FNH is often paler than the surrounding liver parenchyma due to higher glycogen content of its hepatocytes. Fibrous capsule is usually absent. Central fibrovascular scar is a diagnostic feature of FNH (Figure 7.1), seen in approximately two-thirds of the cases; the remainder may require microscopic examination for the identification of thinner fibrous septa. FNH can occasionally be focally or diffusely steatotic, resulting in yellow discoloration mimicking HA or carcinoma. The nodules of a larger FNH may show slight variation from one to another due to focal steatosis, fibrosis, or congestion. FNHs rarely show cholestasis or bile-green discoloration. Large and thick-walled vessels within the central scar or fibrous bands can often be identified on gross examination (Figure 7.2). When a biopsy from the FNH does not include the surrounding noncirrhotic liver parenchyma, it can be mistaken for macronodular cirrhosis. FIGURE 7.1 Characteristic appearance of focal nodular hyperplasia with prominent central fibrous scar and thick fibrous septa separating benign hepatocellular nodules. (Gross photograph.) Microscopically, FNH is composed of normal-appearing hepatocytes, arranged in 1 to 2 cell thick plates, and separated by thick collagenized fibrous bands (Figure 7.3). The hepatocytes can focally show steatosis or contain more glycogen than the surrounding liver parenchyma. The fibrous bands contain ductular reaction and chronic inflammatory infiltrates consisting predominantly of lymphocytes. Neutrophils and scattered eosinophils may be seen in the area of brisk ductular reaction (Figure 7.4). Bridging fibrous bands give rise to nodule formation and often can be mistaken for cirrhotic liver parenchyma in small biopsies or in resection specimens with minimal surrounding noncirrhotic parenchyma (Figure 7.5). FIGURE 7.2 Prominent thick-walled vessels are often grossly visible, embedded within broad fibrous septa, characteristic of focal nodular hyperplasia. (Gross photograph.) FIGURE 7.3 Thick fibrous bands separate nodules of benign liver parenchyma in focal nodular hyperplasia. The liver parenchyma is composed of hepatocytes that are arranged in 1 to 2 cell thick plates. The hepatocytes at the periphery of the nodules appear darker at low magnification due to regenerative features. FIGURE 7.4 Exuberant ductular reaction accompanied by neutrophils are often present in focal nodular hyperplasia. Thick-walled arteries, some eccentrically thickened due to fibromyxoid intimal thickening, are readily observed in the fibrous bands and can be highlighted by elastic stain (Figure 7.6). There is no true portal vein branch, but some ectatic vessels may be seen in the fibrous septa. Focal dilatation of the sinusoids and congestion can occasionally be encountered in those lesions with less than prominent fibrous septa or scar. There are no normal portal tract structures (hepatic artery, portal vein, and bile duct) within the lesion. The hepatocytes in FNH are cytologically normal, but occasional hepatocytes with large cell change or hyperplastic features can be seen. The hepatocytes may also demonstrate increased glycogen content, steatosis, lipofuscin, and iron deposition. A copper stain may demonstrate copper deposits in the hepatocytes adjacent to fibrous bands, which may be the result of the absence of a normal bile duct, leading to cholate stasis (Figure 7.7). In conjuction, these hepatocytes may exhibit feathery degeneration, often accompanied by exuberant ductular reaction at the adjacent fibrous bands with lymphocytic infiltrate, scattered neutrophils, and eosinophils. Epithelioid granulomas can rarely be seen scattered throughout and in the perimeter of FNHs (Figure 7.8). FIGURE 7.5 Needle biopsy specimen has nodular appearance, reminiscent of liver cirrhosis. Notice the absence of a portal tract and the presence of prominent thick-walled vessels as well as the dilated thin-walled vessels. FIGURE 7.6 Eccentrically thickened arteries with fibromyxoid intimal thickening are present and are seen throughout or in the periphery of the lesion, but they are predominantly located in the central fibrous scar of focal nodular hyperplasia. FIGURE 7.7 Rhodanine stain demonstrates brown-orange granules of copper deposits in the cytoplasm of hepatocytes around the central fibrous scar in focal nodular hyperplasia. FIGURE 7.8 Epithelioid granuloma (arrow) can be seen scattered throughout focal nodular hyperplasia. Without sufficient clinical information and imaging findings, the diagnosis of FNH can be difficult in a small liver biopsy specimen, even when the entire lesion is present. The most common biopsy pitfall of FNH is negative diagnosis or misinterpretation for cirrhotic liver parenchyma secondary to biliary or other chronic liver diseases (see Figure 7.5). Needle biopsy of FNH usually shows liver nodules, separated by fibrous bands containing large thick-walled vessels, ductular reaction, and nonspecific lymphocytic infiltrate; and the lack of any remaining portal tract elements as seen in cirrhosis, such as true hepatic artery and its accompanying bile duct. The main differential diagnoses of FNH are HA and HCC. The absence of a grossly obvious central fibrous scar in FNH may cause its resemblance to HA, particularly to the inflammatory or telangiectatic variant of HA (IHA) that had been initially described as FNH in multiple FNH syndromes (Figure 7.9). The predominant features of IHA include congestion or hemorrhage, marked sinusoidal dilatation, chronic inflammation, portal tract-like structures with arteries, and ductular reaction (Figure 7.10). The lack of a central fibrous scar and thick fibrous septa, and the presence of vascular ectasia and marked sinusoidal dilatation favor IHCA over FNH. Immunohistochemical stain for cytokeratin (cytokeratin 7 or 19) is not helpful in distinguishing the two entities, because both lesions contain ductular structures. FIGURE 7.9 Inflammatory hepatocellular adenoma may be multinodular but lacks prominent central fibrous scar. (Gross photograph.) FIGURE 7.10 Portal tract-like structures with unpaired arteries, ductular reaction, chronic inflammation, and dilatation of sinusoids are features of inflammatory hepatocellular adenoma. The differentiation of FNH to conventional HA is more straightforward as conventional HCA lacks fibrous bands and ductular reaction; therefore, cytokeratin 7 or 19 can be helpful in differentiating FNH to conventional HCA. Because of the absence of the bile duct and ductular reaction in conventional HA, cytokeratin 7 or 19 is negative in conventional HA and is positive in FNH (Figure 7.11). Unpaired arteries are present scattered throughout conventional HA with no or very minimal fibrous stroma (Figure 7.12). Other immunohistochemical stains, such as serum amyloid A, c-reactive protein, beta catenin (nuclear), and glutamine synthetase have been reported to be positive in HAs and can be helpful in differentiating FNH to HA (417). A map-like pattern of glutamine synthetase staining is characteristic of FNH (Figure 7.13), while HCA shows diffuse staining. Clinically, the distinction between FNH and HCC sometimes may not be straightforward and there have been a considerable number of reported cases of HCC presenting as FNH or vice versa (418–420). In comparison to FNH, the large majority of HCC occur in patients with underlying chronic liver disease and cirrhosis. HCC is variegated in its gross appearance, often with different areas of hemorrhage, necrosis, and bile production. Highly atypical neoplastic cells with high nuclear to cytoplasmic ratio, arranged in thick plates, occasionally with pseudoglandular formation, are diagnostic of HCC. The fibrolamellar variant of HCC may be grossly similar to FNH with thick fibrous bands and scar (421, 422). The fibrolamellar variant of HCC occurs in younger aged population, similar to the age population of FNH, and with no underlying chronic liver disease. Microscopically, fibrolamellar variant of HCC consists of polygonal neoplastic cells with large vesicular nuclei, prominent nucleoli, and eosinophilic cytoplasm in irregular clusters, embedded in a densely fibrotic stroma with thick lamellar collagen bands (Figure 7.14). Cytokeratin 7 and CD68 immunostains are frequently positive in the fibrolamellar variant of HCC. FIGURE 7.11 Cytokeratin 7 immunostain highlights the presence of ductular reaction in focal nodular hyperplasia. FIGURE 7.12 Unpaired arteries with minimal or no fibrous stroma are seen in hepatocellular adenoma. Other common features of hepatocellular adenoma, such as steatosis and the lack of fibrous septum, are noted in this photomicrograph. FIGURE 7.13 Focal nodular hyperplasia showing map-like pattern of glutamine synthetase staining. Nodular regenerative hyperplasia (NRH) should not be confused with FNH. NRH represents diffuse nodular changes of the liver secondary to heterogeneous intrahepatic circulation, resulting in compression and atrophy of the centrilobular hepatocytes (Figure 7.15). In addition, the fibrous bands that are characteristic in FNH are not seen in NRH. FIGURE 7.14 Fibrolamellar hepatocellular carcinoma occurs in younger patients without underlying chronic liver disease and grossly may be similar to focal nodular hyperplasia, but microscopically the tumor is composed of large polygonal cells with eosinophilic cytoplasm, large nuclei, and prominent nucleoli, separated by lamellated collagenous bands. FIGURE 7.15 Nodular regenerative hyperplasia is a diffuse small nodular change without fibrous septa, resulting in zonal compression of the hepatocytes (arrows). (Reticulin stain.) Large regenerative nodules occur in livers with cirrhosis or chronic liver disease, unlike FNH that occurs in noncirrhotic livers. FNH does not have malignant potential, although, arguably anecdotal cases of fibrolamellar HCC arising in association with FNH can be found in the body of literature (421,422). Unlike HAs, the risk of hemorrhage in FNH is negligible, justifying therapeutic abstention in most cases. Resection is reserved for symptomatic large lesions or for lesions that cannot be distinguished from HA or carcinoma with confidence. While it remains controversial, pregnancy may be allowed and any oral hormone therapy can be continued safely. A large regenerative nodule is a circumscribed nodular neoplastic mimic, composed of a proliferation of hepatocytes without hepatocyte dysplasia in either a liver with cirrhosis or a liver following submassive hepatic necrosis. They are larger than the surrounding cirrhotic nodules and usually are more than 8 mm in diameter. Synonyms include macroregenerative nodule, adenomatous hyperplasia, and adenomatous regeneration. The growing interest in small hepatic nodules reflects the advances in imaging techniques and establishment of surveillance protocols for high-risk populations, which have led to the detection of these lesions in patients with chronic liver diseases, particularly in those with cirrhosis caused by chronic hepatitis B or C virus. Over the years, the nomenclature of small hepatic nodules in cirrhotic liver has been refined into a large regenerative nodule, low-grade dysplastic nodule, high-grade dysplastic nodule, and early HCC (423). In this sequence of events in hepatocarcinogenesis, a large regenerative nodule is considered a benign neoplastic mimic, whereas a low-grade dysplastic nodule and high-grade dysplastic nodule are preneoplastic lesions. Large regenerative nodules are usually found incidentally at autopsy or at the time of transplantation, but they can also be detected by radiologic studies in the course of screening for HCC. Both regenerative and dysplastic nodules display nonspecific imaging features and may mimic HCC. These nodules may show brisk arterial enhancement on CT or MRI, particularly when dysplasia is present in dysplastic nodules. Provided that there is no further suspicion or other nodule of HCC, serum AFP is typically normal or within the low range expected for the underlying cirrhotic liver or chronic liver disease. Large regenerative nodules stand out from the surrounding cirrhotic liver parenchyma by their size and color. Most large regenerative nodules are between 1 cm and 3 cm in diameter. They are surrounded by dense fibrous tissue, are sharply circumscribed, and often bulge out from the cut surface (Figure 7.16). They can be bile stained, steatotic, or paler in color than the surrounding cirrhotic nodules. Large regenerative nodules are best appreciated under low magnification (Figure 7.17). On higher magnification, they are indistinguishable from the surrounding liver parenchyma; hence, needle biopsies invariably result in a negative diagnosis. The hepatocytes are arranged in two cell thick plates as the result of regeneration. The nuclear-to-cytoplasmic ratio remains low throughout the lesion. The hepatocytes may show changes usually seen in cirrhotic liver, including large cell change, oncocytic change, steatosis, cholestasis, cholatestasis, feathery degeneration, or Mallory-Denk hyaline (Figure 7.18). FIGURE 7.16 Large regenerative nodules arise in cirrhotic livers. They are larger and bulge from the surrounding smaller cirrhotic nodules (arrows). (Gross photograph.) FIGURE 7.17 Large regenerative nodule is well circumscribed and contains portal tracts (arrows). FIGURE 7.18 Benign hepatocytes with oncocytic change and large cell change (arrow) are occasionally present in large regenerative nodules. Small cell change is absent, as well as pseudoglandular configuration, thickening of hepatocyte plates (i.e. >2 cell thick plates), increase of nuclear-to-cytoplasmic ratio, and nuclear hyperchromasia. The nodule is surrounded by thick fibrous septa containing blood vessels, dilated lymphatics, ductular reaction, and varying degrees of chronic inflammation. Portal tracts with hepatic arteries, bile ducts, and central venules are often found, particularly at the edge of the lesion. The main differential diagnoses of large regenerative nodules are other (pre) neoplastic lesions in cirrhotic liver, that is, low grade dysplastic nodule, highgrade dysplastic nodule, and well-differentiated HCC (see Table 7.2). Both gross and microscopic features of low-grade dysplastic nodules are often indistinguishable from large regenerative nodules, especially when reviewing needle biopsies. Fundamentally, the distinction between large regenerative nodules and low-grade dysplastic nodules in cirrhotic liver is the absence of a clonal population in large regenerative nodules (423). It was often found to be difficult or impossible to consistently separate these two entities in clinical practice. Therefore, there is currently an understanding that the distinction between these two diagnostic categories cannot be made confidently by morphology alone and does not carry any practical consequences, as long as the nodules lack the features of high grade dysplastic nodule (402).
Hepatocellular or Hepatoid Neoplastic Mimics of the Liver
 ACCESSORY LOBES AND RIEDEL LOBE
ACCESSORY LOBES AND RIEDEL LOBE
 LIVER WITH SUBMASSIVE OR MASSIVE NECROSIS
LIVER WITH SUBMASSIVE OR MASSIVE NECROSIS
INTRODUCTION
FOCAL NODULAR HYPERPLASIA
 CLINICAL AND IMAGING FEATURES
CLINICAL AND IMAGING FEATURES
 PATHOLOGIC FEATURES
PATHOLOGIC FEATURES
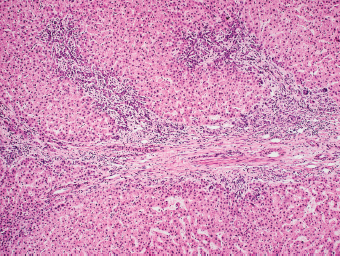
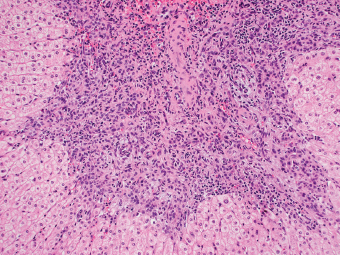
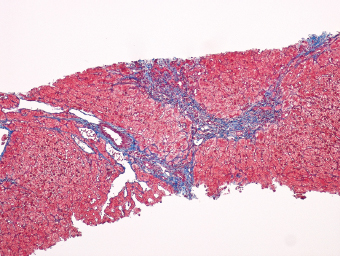
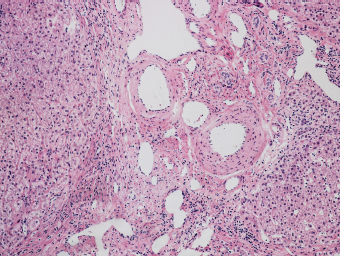
 DIFFERENTIAL DIAGNOSES
DIFFERENTIAL DIAGNOSES

 BIOLOGIC EVOLUTION AND TREATMENT
BIOLOGIC EVOLUTION AND TREATMENT
LARGE REGENERATIVE NODULE
 CLINICAL AND IMAGING FEATURES
CLINICAL AND IMAGING FEATURES
 PATHOLOGIC FEATURES
PATHOLOGIC FEATURES
 DIFFERENTIAL DIAGNOSES
DIFFERENTIAL DIAGNOSES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree