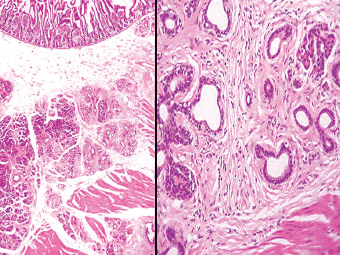
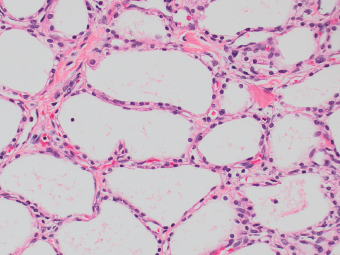
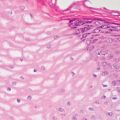
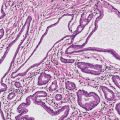
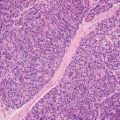
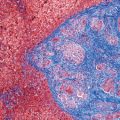
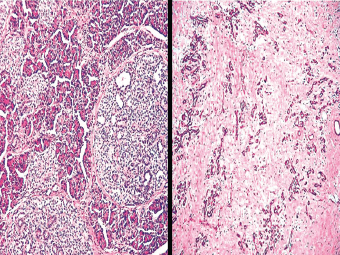
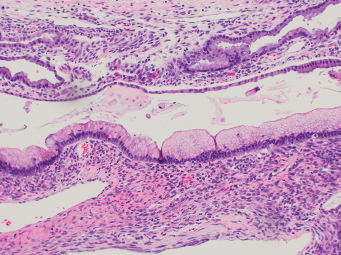
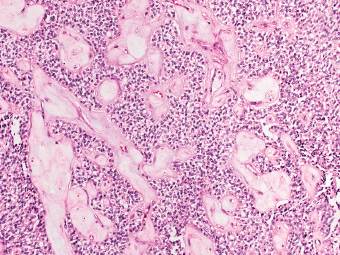
13 The pancreas is an exocrine and endocrine organ, which lies next to the stomach, first and second part of the duodenum, superior mesenteric artery, portal vein, and spleen. Its retroperitoneal location complicates the diagnosis and treatment of neoplasm and neoplastic mimics arising from this organ. The majority of pancreatic neoplasm is adenocarcinoma, which is universally lethal and with few treatment options; therefore, its accurate diagnosis is of importance to reduce the morbidity associated with diagnostic attempts in patients with short survival time. With advanced and accurate imaging studies nowadays, the clinical diagnosis of pancreatic malignancy has been excellent, but there remains the need for confirmatory histologic diagnosis in patients undergoing neoadjuvant treatment, for accurate resection margin assessment in frozen section examination, and for the diagnosis and classification of pancreatic lesions in resected specimens. Neoplastic mimics of the pancreas take the form of tumor, cyst, or tumefactive inflammation on clinical and imaging studies. Pancreatic heterotopia, hamartoma, and teratoma are examples of tumoral neoplastic mimics, while pseudocyst and retention cyst are examples of cystic neoplastic mimics. Tumefactive chronic pancreatitis includes idiopathic and alcoholic chronic pancreatitis, paraduodenal pancreatitis, and autoimmune pancreatitis (AIP). On a microscopic level, glandular and neuroendocrine cell proliferation in chronic pancreatitis are considered neoplastic mimics that are frequently encountered during frozen section or in permanent sections, and sometimes create diagnostic difficulties. Heterotopic pancreatic tissue may be found in the stomach, duodenum, jejunum, ileum (including Meckel diverticula), gallbladder, umbilicus, fallopian tube, mediastinum, and spleen (579–585). The first three sites are, by far, the most common. The incidence of pancreatic heterotopia at autopsy is 1% to 14% of cases and at abdominal surgery roughly 0.2% of cases (586). Many of these heterotopias are incidental findings and a preoperative diagnosis of heterotopic pancreatic tissue is not usually suspected. Because most of these ectopias involve the submucosa of the gut or deeper layers of the stomach and intestines, endoscopic biopsy typically does not sample the abnormal tissue. A frozen section is often requested at the time of surgery. Thus, one must be aware of the anatomic distribution of heterotopic pancreatic tissue so as not to assume that it must represent a metastatic tumor. Heterotopic pancreatic tissue typically presents as a single, firm, yellow-white, lobulated or rounded nodule. Most examples measure roughly 2 cm, with a range of 0.2 to 5.5 cm. Histologic sections reveal a variable cellular composition, most often in the submucosa of the gastrointestinal tract, with the second most common location being the muscularis propria (587) (Figure 13.1). In 10% of cases, the subserosa or serosa is involved; this may cause considerable concern for possible metastatic tumor, but one should recall that the heterotopic tissues usually occupy a solitary site, unlike metastases (Figure 13.2). FIGURE 13.1 Pancreatic heterotopia, seen here in the muscularia propria of the small bowel (left). Both ductal and acinar elements of the pancreas are represented histologically (right). FIGURE 13.2 In contrast to pancreatic heterotopia, which is virtually always unicentric and intramural in the gastrointestinal (GI) tract, metastatic carcinoma typically affects many foci in the GI system and is serosal based. Using one proposed classification for ectopic pancreatic tissue (588), most cases are either type I (acini, ducts, and islets of Langerhans) or type II (acini and ducts), whereas type III (ducts only) is an uncommon variant. Type III may represent adenomyoma, discussed in Chapter 2. Microscopic identification of islets of Langerhans greatly facilitates the diagnosis, but this is not possible in every case. Heterotopic pancreatic tissue is subject to those alterations that are also seen in the main gland, including cystic change, acute and chronic inflammation, fibrosis, hemorrhage, necrosis, and neoplasia. Changes observed in adjacent tissues include fat necrosis, inflammation, hemorrhage, ulceration, and formation of intestinal diverticula. When nests of endocrine cells are prominent in pancreatic ectopy, their association with pancreatic ducts and acini helps to avoid a possible misdiagnosis of carcinoid tumor. Another anatomic variation that may lead to pathologic misinterpretation is the accessory pancreatic ductal system of the major duodenal papilla, which may appear to “invade” the smooth muscle of the duodenal wall (589). In difficult cases, serial sections may clarify the situation. Occasional mitotic figures and nuclear enlargement may be observed in islet cells in heterotopic pancreatic tissue, with associated polyploid DNA content (590,591). These features have no clinical consequence and should not be used to justify a diagnosis of neoplasia. Hamartomas of the pancreas are exceedingly rare (592–597), with relatively few cases having been reported in both children and adult. These masses are well-circumscribed, solid, or cystic (Figure 13.3), and measure up to 15 cm in greatest dimension. Microscopically, ectatic ducts of various sizes are present, surrounded by fibrous connective tissue. Dilated ducts and large irregular lobules of pancreatic acinar tissue are admixed with fibrous tissue and fat (Figure 13.4). Cystic pancreatic neoplasms, including mucinous cystadenoma, microcystic adenoma, and solid pseudopapillary neoplasm of the pancreas (Figures 13.5–13.7), enter into the differential diagnosis with hamartomas (598). Teratomas of the pancreas are likewise exceptionally rare, with less than 50 reported cases (599–601), all concerning individuals who were in the first three decades of life. Almost all patients presented with abdominal or lumbar pain. Fine needle aspiration biopsies of pancreatic teratomas have shown mature squamous cells admixed with neutrophils and keratinous debris (600). Surgically excised masses of this type have been cystic and typically contained hair; they have measured up to 20 cm in greatest dimension (601). Microscopic recognition of at least two germinal tissue lines, potentially including the presence of smooth muscle, hyaline, cartilage, hair, bone, fat, and respiratory or gut epithelium, and neural tissues, is required for this diagnosis. FIGURE 13.3 The macroscopic (and radiological) appearance of pancreatic hamartomas may be that of a solid mass, as shown here, potentially simulating that of a carcinoma. (Photograph kindly supplied by Dr. Gunter Kloppel, München, Germany.) FIGURE 13.4 Ductal, acinar, and mesenchymal tissues are randomly admixed microscopically in pancreatic hamartomas, sometimes with formation of microcystic structures. FIGURE 13.5 Mucinous cystadenoma with characteristic ovarian-type stroma may enter into the diagnosis of pancreatic hamartoma. FIGURE 13.6 Microcystic (glycogen-rich) adenoma of the pancreas comprises relatively small cystic spaces that are mantled by low cuboidal cells with optically lucent cytoplasm. These lesions are intensely reactive for glycogen with the periodic acid Schiff stain, unlike pancreatic hamartomas, which may be included in their differential diagnosis. FIGURE 13.7 Solid pseudopapillary tumor (SPT) of the pancreas enters into the differential diagnosis with pancreatic hamartomas that contain microcysts. However, SPT is composed of a much more monotonous population of lesional cells with no ductal or acinar structures, as expected in hamartomas.
Neoplastic Mimics of the Pancreas
 HAMARTOMAS AND TERATOMAS OF THE PANCREAS
HAMARTOMAS AND TERATOMAS OF THE PANCREAS
 CYSTIC DISEASE IN THE PANCREAS
CYSTIC DISEASE IN THE PANCREAS
 TUMEFACTIVE CHRONIC PANCREATITIS
TUMEFACTIVE CHRONIC PANCREATITIS
 PARADUODENAL (“GROOVE”) PANCREATITIS
PARADUODENAL (“GROOVE”) PANCREATITIS
 PSEUDONEOPLASTIC PROLIFERATION OF ENDOCRINE CELLS IN CHRONIC PANCREATITIS
PSEUDONEOPLASTIC PROLIFERATION OF ENDOCRINE CELLS IN CHRONIC PANCREATITIS
 PRIMARY EPITHELIAL HYPERPLASIAS IN THE PANCREAS
PRIMARY EPITHELIAL HYPERPLASIAS IN THE PANCREAS
INTRODUCTION
PANCREATIC HETEROTOPIA
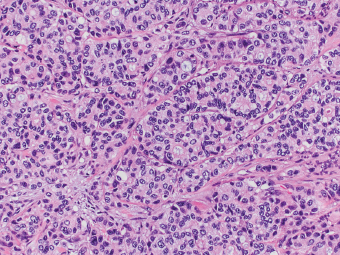
HAMARTOMAS AND TERATOMAS OF THE PANCREAS
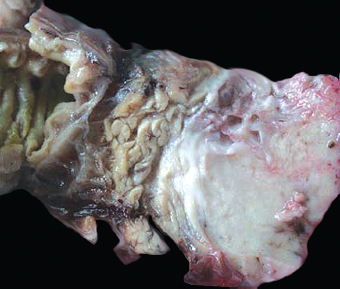
CYSTIC DISEASE IN THE PANCREAS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree