Chapter 54 Hepatitis B Infection and GB Virus C
HEPATITIS B VIRUS
Pathogen
Hepatitis B virus (HBV) is a hepatotropic DNA virus of the family hepadnaviridae. It is a 40–42 nm virion enclosed by a lipoprotein envelope bearing three envelope glycoproteins, or surface antigens.1 Inside the envelope is a nucleocapsid containing the viral genome, as well as a DNA polymerase. The viral genome consists of partially double stranded, relaxed-circular DNA.2 There are eight HBV genotypes (A through H) with distinct geographic distributions and variable response rates to therapy.3,4 The genome possesses four open reading frames: preS-S, preC, P, and X. The presurface–surface (preS-S) encodes three surface antigens including HBsAg, which is the most abundant of the three. The second open reading frame is the precore–core region (preC), which encodes hepatitis B core antigen (HBcAg) and hepatitis B e antigen (HBeAg). HBeAg is secreted into the blood and is used as a marker for unresolved infection, though its function is not well known. PreC mutations result in HBeAg-negative infections which are clinically well-documented, seen in up to 33% of hepatitis B surface antigen (HBsAg) carriers, associated with high rates of relapse, and less favorable outcomes in response to interferon-alpha treatment.1,5 The third open reading frame, or P coding region, encodes a polyfunctional enzyme which is a viral polymerase for DNA synthesis and a reverse transcriptase as well.1 Finally, the X open reading frame encodes viral X protein (HBx) which regulates host and viral gene expression, and has been implicated in the development of hepatocellular carcinoma (HCC).1,6
The Viral Life Cycle
HBV binds to cell surface receptors and is internalized by hepatocytes. Core particles are transported to the nucleus, where their genomes are modified into covalently closed circles (cccDNA). CccDNA is transcribed into mRNA which is transported to the cytoplasm and translated into viral surface, core, polymerase, and X proteins. Subsequently assembled viral particles incorporate viral RNA which is then reverse transcribed into viral DNA.2 Core particles either bud off the host cell membrane to infect other cells, or are transported back to the nucleus where they are converted back to cccDNA and maintain a stable intranuclear pool of templates allowing persistent infection.7 Four antivirals, (nucleoside analogs lamivudine and emtricitabine; nucleotide analogs adefovir and tenofovir) originally developed to inhibit the reverse transcriptase inhibitor of HIV-1, potently inhibit HBV replication. The existence of these dually active drugs provides expanded opportunities for HBV therapy, as well as simplified regimens, in HIV-co-infected patients. Two additional nucleoside analogs, entecavir and telbuvidine, are active against HBV and have no therapeutic role for treatment of HIV.8,9,9a Unfortunately, therapy must be prolonged as cccDNA evades the action of the drugs until the replication phases of the viral life cycle.
Pathogenesis
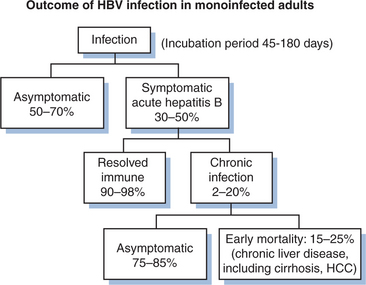
HBV is not directly cytotoxic to hepatocytes. Rather, liver injury occurs as a result of cytotoxic CD8+ T-lymphocyte cell (CTL) responses to the viral antigens that are displayed on hepatocyte surfaces. Aminotransferases rise with hepatocyte death. Not surprisingly, immunocompromised hosts with HBV often have mild acute hepatitis B infections, but high rates of chronicity.2,7 Over 90% of normal adults and 75% of normal infants will resolve acute infection with HBV without therapy. Those who do not resolve acute infection are defined as chronic carriers if they demonstrate serum HBsAg positivity for over 6 months.6
Both cellular and humoral immune responses are implicated in control of infection and development of protective immunity. Anti-HBsAg antibodies (anti-HBS) are produced in response to recombinant envelope antigen and confer protective immunity. These antibodies are found in persons who recover from acute HBV and also in those who have been immunized with recombinant HBV vaccine.1
In HIV-infected patients, as in chronic HBV carriers, desirable anti-HBV cell-mediated immune responses, including CTL (which are believed to play a central role in viral clearance), are blunted. Nevertheless, chronic inflammation from unresolved viral infection generates inflammatory and profibrotic cytokines (such as TNF-alpha and interferon gamma) as well as proteases, free radicals and natural killer cell activity which contribute to the development of chronic liver damage, fibrosis, cirrhosis, and end-stage liver disease (ESLD).6,10 Individuals with chronic HBV infection have a 100 times higher risk of HCC compared to noncarriers.11 Mechanisms for the provocation of HCC by HBV are not well understood. Though cirrhosis is a predisposing factor for HCC, 30–50% of HCC associated with HBV occurs in patients who do not have cirrhosis, and HCC can occur in long-term carriers who have cleared HBsAg. Chronically infected patients should be screened every 6 months for HCC using liver ultrasonography as well as serum alpha-fetoprotein (AFP).6,7 Note that use of both screening modalities is warranted by the imperfect sensitivity and specificity of either test alone; the positive predictive value of AFP is no higher than 30%.6
Epidemiology
Globally, there are some 370 million persons persistently infected with HBV,4,12 with 2–4 million individuals estimated to be co-infected with HIV and chronic HBV.12 HBV is endemic in Asia, sub-Saharan Africa, and the South Pacific.1,6,12 In the United States and Western Europe, there is evidence for chronic HBV co-infection among 6–14% of all HIV-infected persons. Co-infection rates differ according to risk group, with HBV found in 4–6% of HIV-infected heterosexuals, 9–17% of men who have sex with men (MSM), and 7–10% of injection drug users (IDU).12 HBV shares routes of transmission with HIV and hepatitis C (HCV), and is transmitted percutaneously via needlestick injuries, injection drug use, or by blood transfusions, sexual or mucous membrane exposure, and perinatal exposure. However, HBV differs from HIV and HCV in the efficiency by which various exposures result in disease transmission. For example, HBV is 100 times more infectious than HIV from a percutaneous exposure, and HBV is transmitted perinatally and sexually with greater ease than HCV.12
Natural History
The clinical manifestations of acute HBV infection are often clinically undetectable, but may include nausea, vomiting, fatigue, fever, arthralgias, and right-upper quadrant abdominal pain followed by jaundice.13 In the outpatient HIV clinical setting, chronic HBV commonly presents as asymptomatic disease with HBsAg positivity and elevated alanine aminotransferase (ALT). These patients may demonstrate poor tolerance to certain antiretroviral medications, palmar erythema, gynecomastia, cutaneous spider angiomata, and/or hepatomegaly. Later, ESLD manifests with the onset of ascites, jaundice, encephalopathy, coagulopathy, esophageal varices, and/or caput medusa. Extrahepatic manifestations of HBV include vasculitis, glomerulonephritis, and polyarteritis nodosa.1,13 Co-infected patients may be at a higher risk of developing HCC than HBV-monoinfected patients,14,15 but screening recommendations for HCC in this population are similar to those in monoinfected persons: AFP and hepatic ultrasound should be performed every 6 months.7,13,15
Most (95%) acute HBV infections are resolved by the host immune response (Fig. 54-1). Initially there are high levels of serum HBV DNA, and HBeAg is measurable. In those who resolve infection, HBeAg is eventually lost; antibody to HBeAg (anti-HBe) appears, with a concomitant reduction of HBV DNA to fewer than 105 copies/mL, normalization of aminotransferase levels, and reduction of necroinflammation.6 HBeAg to anti-HBe conversion is frequently preceded by an ALT flare.16 However, fulminant or chronic persistent hepatitis may develop due to incompletely defined host and viral factors. Persistence of HBV occurs more often with the HBV/Ae genotype than others. Fulminant hepatitis is associated with HBV/Bj accompanied by lack of HBeAg and a precore mutation.4 In HIV-negative patients with chronic HBV, 25% will develop serious complications of HBV during their lifetime.7
Some 0.5–2.5% of chronic carriers clear HBsAg yearly and then develop anti-HBs.16,17 Up to half of these people will still have low levels of HBV DNA detectable by PCR, the significance of which is not well understood. About 20% of HBV-infected patients will develop cirrhosis as a result of the chronic carrier state.2 The 5- and 10-year survival rates for HBV patients with compensated cirrhosis are 84% and 68%, respectively. The annual incidence of HCC in HBV carriers with cirrhosis ranges from 3% to 10%.16
When a patient with HBV infection worsens, or when a test for HBeAg is negative in the presence of active liver disease, hepatitis D (HDV) infection should be considered. HDV is an RNA-containing defective passenger virus which cannot replicate in the absence of HBV. The results of treatment in HBV/HDV-infected persons have been disappointing.7 There is no specific therapy for HDV, which is unfortunate, because disease progression is more rapid with combined infections than with HBV infection alone.1
Impact of HIV on the Natural History of HBV
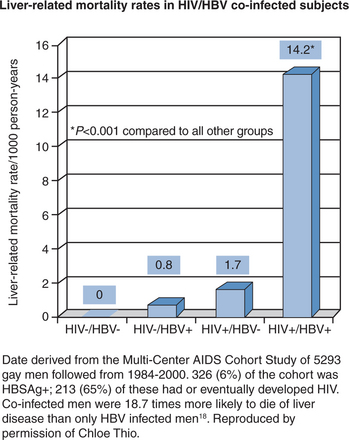
Patients with HIV/HBV infection have substantial liver-related mortality (Fig. 54-2) Co-infected persons have more severe liver disease and higher liver-related mortality rates than do HBV-monoinfected persons.7,18,19,9,20 Analysis of the multicenter AIDS Cohort Study found a 17 times higher liver-related mortality rate in co-infected patients compared with HBV-monoinfected individuals.18 HIV/HBV-co-infected patients also have higher levels of HBV DNA and lower rates of HBeAg seroconversion.6,7,21,22 Moreover, the tolerability of HIV medications can be adversely affected by the presence of HBV co-infection.7,23–25 The incidence of serious drug-related hepatotoxicity in HIV/HBV-co-infected persons can be as much as nine times higher than for HIV-monoinfected persons.26 Antiviral drugs can cause liver injury directly, or they can lead to immunologically mediated liver injury by effecting immune reconstitution in immunocompromised individuals.7,27
With the advent of highly active antiretroviral therapy (HAART), and greatly improved survival of patients with HIV infection, chronic liver disease due to hepatitis B or C co-infection has now become a major cause of morbidity and mortality for patients with HIV.28–31 It is important, therefore, to screen all HIV-infected persons for viral hepatitis co-infection, and to provide hepatitis A and B vaccines to those who are not immune.13,7 The presence of HBV infection at initial screening of an HIV-infected person will critically affect the timing and choice of antiretroviral regimen,32 as well as long-term clinical management (see section on Treatment).
Diagnosis
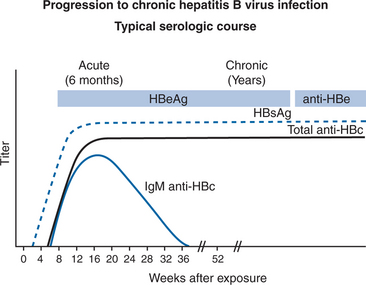
The optimal testing strategy for HBV in HIV-infected persons is not well-defined.13 In screening HIV patients, it is important to understand the serological markers that distinguish between acute, chronic, and resolved HBV infection. One must also remember the distinct pattern displayed by individuals with the preCore mutation, and that immunocompromised hosts sometimes display aberrant serology. The usual markers of acute infection include hepatitis B core IgM, HBsAg, HBeAg, and HBV DNA.105 copies/mL (Figs 54-3 and 54-4).1 Individuals with chronic infection are so defined if they remain HBsAg positive for greater than 6 months. The other markers of chronic infection are HBeAg, anti-HBe antibody negativity, HBV DNA.105 copies/mL and (usually) elevated ALT. Individuals chronically infected with the HBV preCore mutant will be HBeAg negative with elevated ALT and elevated HBV DNA levels. Markers of resolved infection are anti-HBe antibody positivity, negative HBeAg, loss of HBsAg, normal ALT, and HBV DNA < 105 copies/mL. The aberrant serology sometimes noted in HIV-infected persons is characterized by the presence of HBV DNA in persons without HBsAg, in whom anti-HBc is the only serological marker of infection.33–36
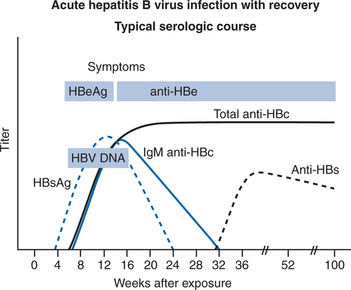
Figure 54-3 Acute hepatitis B virus infection with recovery: Typical serologic course.
Reproduced from the CDC.
Treatment
HBV-specific treatment is recommended for co-infected patients with a positive HBeAg or HBV DNA levels >105 copies/mL and liver disease (elevated ALT and/or fibrosis seen by liver biopsy).7 Ideally, the goal of therapy should be the resolution of infection as defined by the disappearance of HBsAg and the appearance of anti-HBs (antibodies against HBsAg). However, this goal is rarely achieved as cccDNA evades available drugs. Failing cure, patients can still benefit from the more modest goal of halting the progression of fibrosis while preventing ESLD or HCC. Antiviral therapy can be used to maximally suppress HBV replication for as long as possible. There are five parameters that may be monitored prospectively for treatment response: HBV DNA, HBeAg, HBsAg, ALT, and liver histology.7 A currently accepted surrogate marker for successful anti-HBV treatment is clearance of serum HBV DNA. Alternatively, if HBV DNA PCR is not available, anti-HBe antibodies are considered markers of successful treatment response in individuals who were HBeAg-positive prior to therapy.8
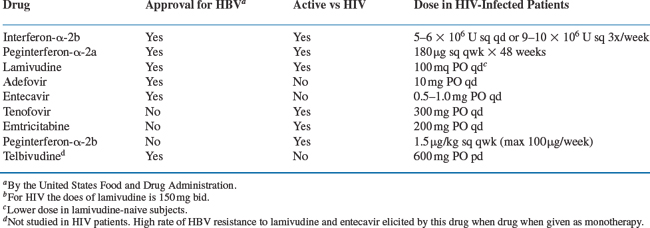
There is no uniform treatment recommendation for the management of HBV in the setting of HIV.13 Because of limited data about the safety and efficacy of chronic HBV treatment in co-infected persons, current guidelines are based on consensus opinion.7 The approved drugs for treatment of HBV include pegylated interferon-alpha 2a, as well as non-pegylated interferon alpha 2a or 2b37 (nearly all practitioners choose the pegylated formulation of interferon alpha due to more favorable outcomes and dosing schedule)38–40 the nucleoside analogs lamivudine or entecavir,5,14,41–45 or the nucleotide analog, adefovir dipivoxil.8,46,47 Not approved for HBV by regulatory agencies, but clearly efficacious are the nucleotide analog tenofovir (approved for HIV, and closely related to adefovir)47–52 and the nucleoside analog emtricitabine (also developed for HIV, and related to lamivudine)53–55. (Telbivudine has not been studied in co-infected patients to date.) Of these active drugs, lamivudine, emtricitabine, and tenofovir have dual activity against HIV and HBV. While entecavir was initially not noted to have anti-HIV activity, there are a few cases reported in which coinfected patients treated with entecavir monotherapy had reductions in HIV viremia and subsequently developed the HIV resistance mutation M184V, conferring HIV resistance to lamivudine and emtricitabine. While the significance of these few reports is not yet well understood, experts advise the avoidance of entecavir monotherapy in coinfected persons.56 When used in co-infected persons, entecavir should be given with a full HAART regimen.56a A summary of therapeutic options for HBV in HIV/HBV-co-infected patients, including regulatory agency approval status and dosing schedules, is listed in Table 54-1.
The first drug approved for chronic hepatitis B in monoinfected persons was interferon-alpha 2b.9 For monoinfected persons, interferon alpha is more efficacious in HBeAg1 than in HBeAg2 persons.7 In HIV/HBV-co-infected patients, the response rate to therapy with interferon (14.3%) is inferior to that seen in HBV-monoinfected patients, who achieve a 36–45% remission rate after 4 months of treatment.1,9,57 Moreover, interferon therapy requires subcutaneous injections and presents many side effects. Pegylated interferon alpha requires less frequent injections, and is more effective against HBV than nonpegylated interferon.9,58 While rigorous trials of this drug in co-infected patients are lacking, some experts feel that certain variables favor the treatment of HBV/HIV-co-infected patients with pegylated interferon. These variables include: HBeAg positivity, elevated aminotransferases, high CD4+ T-lymphocyte counts, absence of advanced liver disease, no need for HAART, and absence of psychiatric disorders.8 Co-infected patients are more commonly treated with oral medications because dual-purposedrugs directed against both viruses permit a tolerable, simplified regimen. Monotherapy for HBV with oral nucleoside analogs presents a significant risk of drug resistance development. For example, mutations in the active site of HBV polymerase/reverse transcriptase targeted by lamivudine (YMDD mutations), develop at a rate of 15% per year. In HIV/HBV-co-infected patients, these mutations are more rapidly selected, at a rate of 50% after 2 years, and 90% after 4 years.8,59 YMDD mutations can also be elicited by emtricitabine, which is closely related to lamivudine. Fortunately, the nucleotide analogs tenofovir and adefovir have demonstrated in vivo activity against YMDD mutant viruses.7
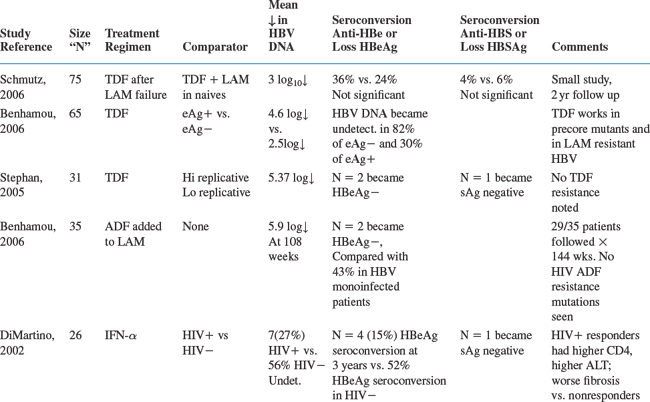
Table 54-2 summarizes some of the larger trials forco-infected patients.
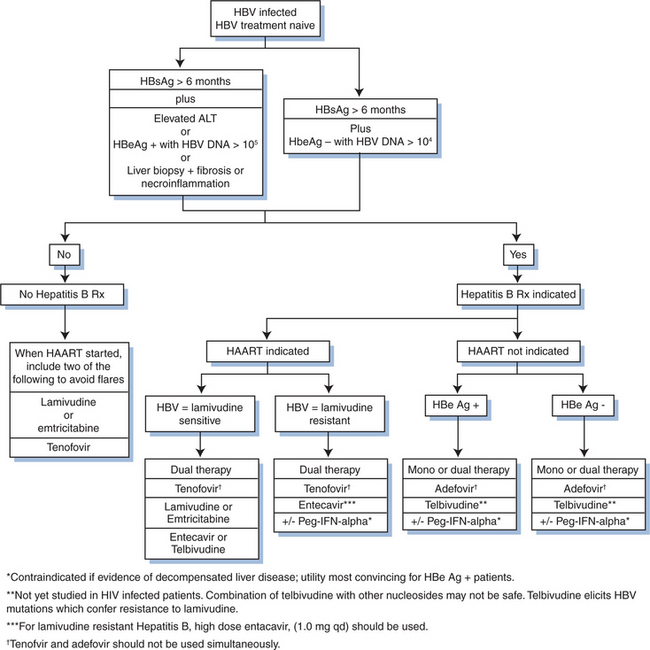
A currently accepted algorithm for treatment of HBV in co-infected patients starts with serological assessment of patients who are HBsAg positive (Fig. 54-5). These individuals should be screened for HBeAg, anti-HBe, and quantitative plasma HBV DNA PCR. A liver biopsy is not required, but may be considered if there is no immediate indication for HIV treatment, in patients with normal ALT, or with low levels of HBV DNA (104 copies/mL). Furthermore, liver biopsy may be useful if alternate, co-existing morbidities (like steatosis or opportunistic infections) are suspected.
In treatment-naive patients whose HIV disease does not warrant initiation of HAART, HBV treatment should consist of pegylated interferon or adefovir. The guiding principle is to avoid monotherapy using the dual purpose drugs (lamivudine, emtricitabine, or tenofovir) in this setting, to prevent development of HIV resistance mutations to these drugs. For HBeAg-negative individuals who do not require HAART, adefovir monotherapy may be initiated.8 Pegylated interferon may be used for e-antigen negative cases, however, the response rate is less favorable than for e-antigen positive cases, with a higher rate of relapse. Using adefovir in co-infected individuals theoretically could lead to cross-resistance to tenofovir, as adefovir does inhibit HIV replication at higher doses. However, such cross-resistance has yet to be demonstrated clinically.9,60 If entecavir is used in coinfection, it should be used in the context of a full HAART regimen to avoid HIV resistance mutation M184V.
In patients who require simultaneous treatment of HIV and HBV, consensus opinion suggests that HAART should be initiated including tenofovir with either lamivudine or emtricitabine in addition to a third HIV specific drug.8,9 This opinion prevails despite the existence of a black box warning stating that tenofovir is not indicated for the treatment of chronic hepatitis B virus infection, and that the safety and efficacy of tenofovir have not been established in patients co-infected with HBV and HIV. Severe exacerbations of HBV have been reported in patients who are co-infected with HBV and have discontinued tenofovir.9 The rationale for the consensus opinion relates to the known high rate of development of HBV resistance to lamivudine monotherapy in co-infected individuals, as well as clinical evidence of potent anti-HBV activity by tenofovir.9,56 There is at least one clinical trial in HIV/HBV-co-infected individuals examining the strategy of tenofovir with lamivudine compared with either drug alone among lamivudine-naive or experienced patients. This small study (N = 59), compared dual to monotherapy for HBV. At week 24 among naive subjects, combination therapy with tenofovir was superior to monotherapy, and there was no difference between the tenofovir versus lamivudine monotherapy arms.61 The study also supports either adding or switching to tenofovir in lamivudine-experienced patients who have lamivudine resistance. If only HIV treatment is indicated, one should include at least one anti-HBV drug in the HAART regimen to avoid flares in aminotransferases that can be seen in co-infected persons undergoing HAART-mediated immune reconstitution.8 Monotherapy with dual purpose drugs (emtricitabine, lamivudine, and tenofovir) should be avoided to prevent the selection of HIV resistance mutations.56
Patients with HBV viremia who have been taking lamivudine-containing HAART without a second HBV active drug for more than 2 years may be presumed to have lamivudine resistance mutations. These individuals have been shown to benefit from the addition of tenofovir,61 but not emtricitabine (due to cross resistance with lamivudine). They are also likely to benefit from the addition of adefovir, which will treat HBV without altering HIV viral load. There is concern for elicitation of HIV resistance mutations from the use of entecavir, as previously noted.
In the absence of clinical data to guide treatment duration, and especially in settings where access to serologic testing is not always possible, one approach is to continue HBV therapy indefinitely in HIV-infected persons. There are documented cases of fulminant hepatitis and liver enzyme flares in co-infected patients whose lamivudine-containing regimens were changed to regimens without an HBV active drug.62,56 Published guidelines on management of opportunistic infections in HIV recommend that HBeAg positive, HIV/HBV-co-infected patients who become HBeAg negative and anti-HBeAg positive should be treated for a minimum of 1 year beyond HBeAg seroconversion.13 For individuals who are HBeAg negative at the outset, there is no information on optimal treatment duration.13
Several newer drugs are being investigated for HBV treatment in clinical trials. These include nucleoside analogs telbivudine (now approved for HBV monotherapy), clevudine, elvucitabine, and valtorcitabine, none of which possess anti-HIV activity, as well as one more nucleoside analog, DAPD, which is active against both viruses.8 Regardless of the regimen used, all chronically HBV infected persons should be counseled to avoid alcohol consumption to minimize further liver damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree