Epithelial tumors
Hepatocellulara
Benign and tumorlike lesions
Hepatocellular adenoma (adenomatosis)
Focal nodular hyperplasia
Macroregenerative nodule
Precancerous lesion
Dysplastic nodules
Malignant lesion
Hepatoblastoma
Epithelial subtypes
Pure fetal with low mitotic activity
Fetal, mitotically active
Pleomorphic, poorly differentiated
Embryonal
Small-cell undifferentiated
INI1 negative
INI1 positive
Epithelial mixed (any/all above)
Cholangioblastic
Epithelial macrotrabecular pattern
Mixed epithelial and mesenchymal
Without teratoid features
With teratoid features
Hepatocellular carcinoma
Classic HCC
Fibrolamellar HCC
Hepatocellular neoplasm, NOSb
Bile duct
Benign
Bile duct adenoma /hamartoma, others
Malignant
Cholangiocarcinoma
Combined (hepatocellular cholangiocarcinoma)
Mesenchymal tumor
Benign
Vascular
Infantile hemangioma
Mesenchymal hamartoma
PEComas
Malignant
Embryonal sarcoma
Rhabdomyosarcoma
Vascular
Epithelioid hemangioendothelioma
Angiosarcoma
Other malignant tumors
Undefined tumors
Malignant rhabdoid tumor
INI1 (INI1 mutation)
INI1+
Nested stromal-epithelial tumor
Others
Germ cell tumors
Teratoma
Yolk sac tumor
Desmoplastic small round cell tumor (DSRCT)
Peripheral primitive neuroectodermal tumor (pPNET)
Metastatic(and secondary)
Solid tumors (neuroblastoma, Wilms’ tumor, others)
Acute myeloid leukemia (M7)
8.1.2 Requirements of Tissue Sampling and Delivery for Diagnosing Hepatic Tumors in Children
Diagnostic biopsy must be provided with the age, serum AFP, imaging data, and clinical information of the children, aiming at obtaining adequate tumor tissue for histological diagnosis and classification and obtaining fresh juncture tissue between the tumor and normal liver tissue for cytogenetics and of molecular biology.
Biopsy must be conducted before the start of chemotherapy, because chemotherapy of hepatic tumors in children may have impacts on the differentiation and induction which is still not clear. The international institutions are endeavoring to collect large quantities of pre- and post-chemotherapy data for these tumors for comparison, study, and analysis; thus, the present histological classifications are applied only to the pathodiagnosis of pre-chemotherapy specimens.
At present, the guidelines of children’s oncology group (COG) recommend sampling methods to obtain the liver tumors which are as follows: percutaneous needle aspiration, laparoscopic or open needle biopsy, or wedge biopsy. According to the condition of pediatric patient, the tumor size, and the location, different choices can be made. Because of the heterogeneity commonly found in hepatic tumors in children, the adequacy of the biopsy specimens is crucial for accurate pathodiagnosis and typing. And uncertainties exist in aspects of sampling, including how much tissue can reflect the whole tumor in biopsy and how representative of the biopsy is. Thus, the guidelines require ultrasound or imaging-guided sampling according to the different regions of the tumor tissue via coarse needle biopsy, obtaining no less than ten strips, and each strip of tissue should be not less than 1 cm × 0.3 cm in size. If possible, take a small amount of adjacent nontumor liver tissue for inspection. And in order to avoid dissemination of the tumor inside the needle tract, it is recommended to use coaxial needle, which achieves removal of tumor tissue and tissue in the needle at the same time. Intraoperative frozen pathodiagnosis should be avoided, for frozen sections are generally used only for margin assessment. Fine needle aspiration is not suitable for tumor diagnosis, since the obtained cells or tissues are far from enough for the assessment of the tumor.
Biopsy or resected specimens should be sent to the Department of Pathology freshly, and if tumor specimens are sufficient in amount, molecular biology of cytogenetics and tests can be done when necessary, which is important in the study of the mechanisms and the improvement of the treatment of tumors. Hopefully, in the future study, some prognostic index in molecular biology can be beneficial in deciding whether to reduce the drug in the treatment to avoid toxicity or to reduce the intensity of treatment to improve prognosis. According to the experience of COG, the diagnosis of simple fetal-type HB with low mitotic activity in the resected specimen is very important in the prognosis and treatment, which can prevent these children from chemotherapy. Total removal of the tumors can cure the children, so first-stage operations should be increased as possible, not only to reduce unnecessary chemotherapy but also for a better understanding of small-cell undifferentiated composition in the tumor and evidence and experience on deciding the lowest proportion of tumor components to have an impact on the prognosis.
In order to ensure the access to poorly differentiated areas (such as small-cell undifferentiated type) in resected tumor, sampling should be conducted in each different regions of the tumor. The total number of samples should be at least equal to or larger than the number of centimeters of the maximum diameter of the tumor on the tumor resection specimens. These sampling parts should include ink-marked margins and involved large blood vessels (such as the portal vein, hepatic vein, and inferior vena cava), and both the sampling position and the infiltration degree of large blood vessels are required to be recorded in detail. While tumor size and location of certain hepatic segment should be recorded in cases with multifocal tumors, the conditions of tumor cells growing in small blood vessels, which are either in or outside the tumor, are required to be reported.
For post-chemotherapy resected specimens, pathological examination is mainly aimed at accurately describing and comparing the changes before and after chemotherapy (such as the ratio of tumor necrosis and the type of residual viable tumor) and molecular biological characteristics related to the survival tumor cells (such as expression of drug-resistant genes).
8.2 Malignant Hepatic Tumors in Children
8.2.1 Epithelial Tumors
8.2.1.1 Hepatoblastoma (HB)
Pathogenesis and Mechanism
HB is the most common pediatric hepatic malignant tumor, accounting for 1% of all malignant tumors found in children under 15 years old, and nearly 90% of them are discovered in young patients under the age of 5, 70% in patients under 2 years old, and 4% at birth. Most of hepatic tumors in children are sporadic, and the male-to-female ratio is 1.4–2: 1, and some cases are related to familial tumor syndrome (Table 8.2), metabolic diseases, individual susceptibility, and so on, while the exact etiology is still unknown. In recent years, the incidence of HB has been rising, possibly related to the increased survival rate of low birth weight infants, as it has been reported that the lower the birth weight, the higher the risk of developing HB , and the risk values of developing HB in patients with birth weight of <1000 g, 1000–1500 g, and 2000–2500 g are 15.64, 2.53, and 1.21, respectively [3]. Other hereditary syndromes (Beckwith-Wiedemann syndrome, Li-Fraumeni syndrome, familial adenomatous polyposis, Edward’s syndrome, etc.), chromosome abnormalities (2, 8, 13, 18, 21 trisomy, etc.), environmental factors (parents with exposure to metal, petroleum products, paint, acetaminophen, tobacco, etc.), and some of the common chromosome recessive hereditary metabolic diseases (such as glycogen storage disease type I) can increase the risk of HB . The molecular mechanism and tumor signal transduction pathway activation play a role in the genesis of HB , mainly involving pathways such as Wnt pathway, hepatocyte growth factor/C-Met (PI3K/AKT and MAPK) pathway, insulin-like growth factor (IGF) pathway, sonic hedgehog (SHH) pathway [12], Notch pathway [13], and so on.
Table 8.2
Common hepatic tumor-related hereditary diseases in children
Disease | Classification | Chromosome | Gene |
|---|---|---|---|
Trisomy 18 | Hepatoblastoma | 18 | |
Beckwith-Wiedemann syndrome | Hepatoblastoma | 11p15.5 | p57KIP2 |
Hemangioendothelioma | |||
Familial adenomatous polyposis | Hepatoblastoma , adenoma, hepatocellular carcinoma , cholangioadenoma | 5q21,22 | APC |
Li-Fraumeni syndrome | Hepatoblastoma , undifferentiated sarcoma | 17p13 | P53 |
Glycogen storage disease type I | Hepatoblastoma , adenoma, hepatocellular carcinoma | 17 | Glucose-6-phosphatase |
Hereditary tyrosinemia | Hepatocellular carcinoma | 15q23–25 | Fumaryl acetoacetic acid lyase |
Alagille syndrome | Hepatocellular carcinoma | 20p12 | Jagged-1 |
Other familial cholestasis syndromes | Hepatocellular carcinoma , cholangiocarcinoma | 18q21–22, 2q24 | FIC-1, BSEP (ABCB11) |
Neurofibromatosis | Hepatocellular carcinoma , malignant neurinoma, hemangiosarcoma | 17q11.2 | |
Ataxia telangiectasia | Hepatocellular carcinoma | 11q22–23 | ATM |
Fanconi anemia | Hepatocellular carcinoma , hepatic adenoma, fibrolamellar carcinoma of the liver | 20q13.2–13.3 | FAA, FAC\BRCA2 |
1q42, 3p, others | |||
Tuberous sclerosis | Angiomyolipoma | 9q34, 16p13 | TSC1, TSC2 |
Clinical Features
Among the 50 cases of HB treated by surgical resection and pathodiagnosis in Eastern Hepatobiliary Surgery Hospital, Second Military Medical University during the period from January 1983 to December 2013, the patients were under the age of 18 years with a male-to-female ratio of 2.57: 1 (36:14) and an average age of 43.6 (6–210, median 27.5) months, of which patients under 4 years old account for 64%, while 74% of the patients go to the hospital because of abdominal pain or accidentally found abdominal mass, and 24% are found in physical examination. In a case affecting a Chinese female infant who developed jaundice 3 days after birth, abdominal huge mass was found and resected 40 days after birth, which was located in the left lobe of the liver and was mixed type with a size of 10 cm × 8 cm. HB patients often manifest abdominal swelling, secondary hepatomegaly, weight loss or gain, abdominal pain, and digestive tract symptoms, such as nausea, vomiting, and decreased appetite, and jaundice is found in only 5% of the patients. The tumors are located in deep parts and the child cannot express itself, resulting in huge or dissemination of the tumor at primary diagnosis; irregular mass can be palpated in the right upper abdomen in physical examination, which is hard even exceeding midline and pelvic brim in some cases. Some cases are associated with some clinical syndrome associated with developmental malformations, and a few have sexual precocity or androphany due to secretion of gonadotropins by the tumor, e.g., increased levels of serum chorionic gonadotrophin and testosterone. Seventy percent of the HB patients may be associated with anemia, 50% with thrombocytosis, and 80–90% with significantly increased serum alpha-fetoprotein (AFP), while undifferentiated small-cell HB and few fetal HB have normal or slightly increased serum AFP. The AFP level shows parallel changes with the disease course and can be used to evaluate and assess the residual of the tumor and reaction of postsurgical chemotherapy and monitor tumor recurrence. Since AFP is produced by the fetal liver, neonatal serum AFP level can be extremely high, and the AFP concentration in normal full-term infants can be up to more than 100,000 ng/ml which gradually decreases months after birth to <10 ng/ml at 1 year old. So for infants <1 year old, careful evaluation and assessment should be made. All the 50 cases of HB diagnosed in Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, had no previous history of viral hepatitis, 78% of which have serum AFP of ≥1000 μg/L. And distal metastasis at the time of diagnosis was found in 20% of HB cases, with the lung as the most common metastatic site, and other metastatic sites include the bone, brain, eye, ovary, and so on.
Controversy remains in whether adults can develop HB , and 2010 WHO classification of hepatic and intrahepatic bile duct tumors did not describe adult HB , but there have been 50 cases of adult HB in the literature. Compared to pediatric HB , both adult and pediatric HB contain similar numbers of tumor nodules, but pediatric type tends to involve the right lobe of the liver (55–60%), while adult cases often involve both the left and right lobe in a similar proportion. About 90% of pediatric cases have significantly increased serum AFP level, while adult HB displays normal serum AFP levels, and cases with AFP > 200 μ g/l only account for 25%. Pulmonary metastasis can be found in pediatric HB , while adult HB may present metastasis in the lymph nodes and visceral organs including the lung. The prognosis of adult HB aged older than 45 years old is markedly poorer than that of patients under 45 years old.
Pathological Staging
- 1.
Pre-/posttreatment tumor extension (PRETTEXT/POSTTEXT) staging by the International Childhood Liver Tumors Strategy (SIOPEL). This system is based on the segmental anatomy of the liver, with the basic index, e.g., the number and extension of tumors involving four hepatic lobes (left lateral lobe, left internal lobe, right anterior lobe, and right posterior lobe), without any involvement of histopathology. It is widely used in the formulation of therapeutic strategy and evaluation of the prognosis (Table 8.3), and the 2005 edition added the annotation of invasion extension. The recommendations made by COG suggested surgical guidelines which include the following: (1) PRETEXT stages I and II at diagnosis, lobular or segmental resection of the liver can be conducted; (2) POSTTEXT stage II or III, without vascular invasion grossly (V−, P−), lobular or tri-segmental resection of the liver after neoadjuvant chemotherapy is suitable; (3) POSTTEXT stage III, with vascular invasion grossly (V+, P+); or POSTTEXT stage IV, extremely complex surgical resection or liver transplantation after neoadjuvant chemotherapy can be adopted [4–7].
Table 8.3
PRETTEXT/POSTTEXT staging of hepatoblastoma
Stage
Characteristics
Stage I
Tumors are confined in one hepatic lobe (segments 2 and 3 in the left lateral lobe, or segments 6 and 7 in the right posterior lobe), with no invasion of three adjacent lobes
Stage II
Solitary or multiple nodules involving one or two hepatic lobes, with no invasion of two adjacent lobes
Stage III
Huge tumors with involvement of three hepatic lobes except one adjacent lobe with several subtypes, including right anterior tumor involving left internal lobe or multinodular tumor with exemption of involvement of the right anterior and/or left lateral lobe or left internal and right anterior lobes
Stage IV
Huge tumors or multinodular tumors involving all four hepatic lobes
Annotation
V
Involvement of the inferior vena cava or all three major hepatic veins (right, middle, and left)
P
Left and right branches of portal vein
E
Extrahepatic involvement, such as the diaphragm, abdominal wall, stomach, colon, etc.
M
Distal metastasis (commonly seen in the lung, barely seen in the bone and brain)
C
Caudate lobe
F
Multinodular tumors
N
Involvement of lymph nodes
Of the 50 cases of pediatric HB diagnosed and excised in Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, cases at PRETEXT stages I, II, III, and IV account for 34%, 52%, 12%, and 2%, respectively. All these 14 cases affecting female patients (100%) were stage I–II, while of the 36 cases affecting male patients, 29 cases were (81.6%) stage I–II, with worse PRETEXT staging in male HB patients than females.
- 2.
North American children’s oncology association risk stratification for HB . This system grades the risk of HB into four stages from low risk to high risk, involving multiple index related to surgery and pathology (Table 8.4) and can be referred to and applied in the clinical practice [4].
Table 8.4
North American children’s oncology association risk stratification for hepatoblastoma
Stage
Features
Stage I
Extremely low risk: simple fetal type, tumors resected at diagnosis, PRETEXT stage I or II, hepatic segmental resection, or standard lobular resection
Stage II
Low risk: all histology status, tumors resected at diagnosis, PRETEXT stage I or II
Stage III
Moderately low risk: PRETEXT stage III or IV; extrahepatic tumors (invasion of hepatic vein/portal vein/extrahepatic tissues); undifferentiated small-cell type
Stage IV
High risk: metastasis at diagnosis, serum AFP < 100 ng/ml
- 3.
Maibach et al. [8] (2012) included serum AFP concentration, metastasis, PRETEXT staging, age, and pathological parameters to form a stratification diagram of influencing factors for PRETEXT staging and prognosis of HB , dividing the disease-free survival (DFS) of HB patients into three groups: PRETEXT stage I/II/III, without any other risk factors, 3-year DFS reaches 90%; PRETEXT stage IV, and/or multinodular tumors, and/or >5 years old, and/or AFP ≥ 1.2 × 106 ng/ml, 3-year EFS reaches 70%; and undifferentiated small-cell tumor, and/or AFP<100 ng/ml, and/or metastasis, 3-year DFS is 49%.
Gross Features
About 80% of HB lesions are solitary masses, and 58% are located in the right lobe and 15% located in the left lobe, and 27% of the cases involve the left and right lobe. Single huge lesion across the midline or bilobular multifocal lesion can also be found. The tumors are 5–20 cm in diameter, slightly lobulated, or protrude on the surface of the liver. The cut sections show well-defined borders between the tumor and the surrounding liver tissue with a pseudocapsule separating the two, and the tumors are grayish white, brown, or green, commonly seen with hemorrhage and necrosis. Of the 50 cases of surgically resected HB diagnosed in Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, the average diameter was 11.2 (2.5–20) cm (Figs. 8.1 and 8.2).
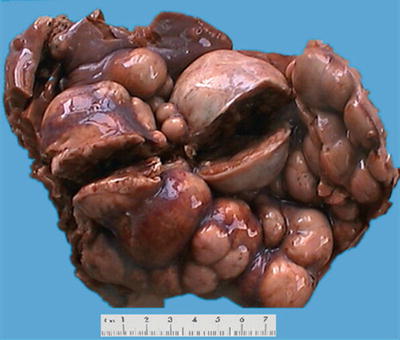
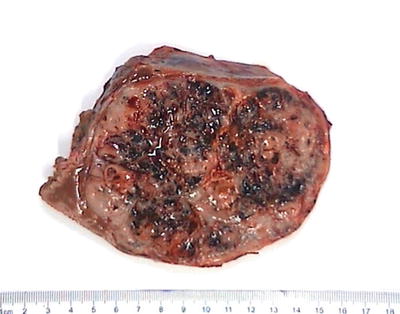

Fig. 8.1
Hepatoblastoma . The tumor presents multi-nodular in shape

Fig. 8.2
Hepatoblastoma . The cut section shows well defined borders, and the tumors are gray-white with extensive hemorrhage and necrosis
Histological Classification
In 2011 Los Angeles COG Liver Tumor Forum edited the classification of HB , mainly including the following types (Table 8.5):
- 1.
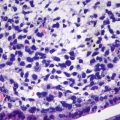
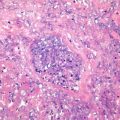
Well-differentiated fetal type (WDF). Also known as pure fetal HB with low mitotic activity, this type accounts for 7% of all epithelial HB . The tumor cells are smaller than nonneoplastic hepatocytes and similar to the size of fetal hepatocytes, with a diameter of 10–20 μm, and are consistently round and cubic in shape. The nuclei are small, round, and center located, with thin chromatin, clear cell membrane, and nonobvious nucleolus. Some cells contain varying amounts of glycogen or lipid resulting in eosinophilic or translucent cytoplasm, which are called as “DAR cells” and “bright cells” (Fig. 8.3). The tumor cells are arranged in cords or thin trabecules, with 2–3 cellular layers and mitotic figures <2个/10HPF (Fig. 8.4). Extramedullary hematopoiesis (EMH) can be easily found. The diagnosis of WDF is only for pre-chemotherapy completely resected specimens, and according to the treatment guidelines of COG, this type of HB does not need postsurgical chemotherapy after complete resection, with tumor-free survival rate of 100%.
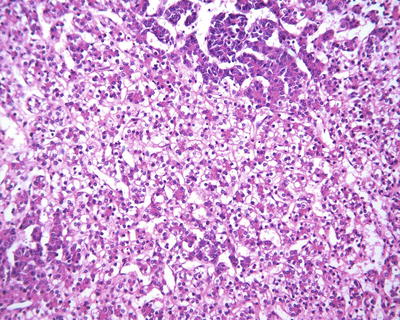
Fig. 8.3
Fetal hepatoblastoma , Some cells contain varying amounts of glycogen or lipid resulting in eosinophilic or translucent cytoplasm, which are called as “dark cells” and “bright cells”
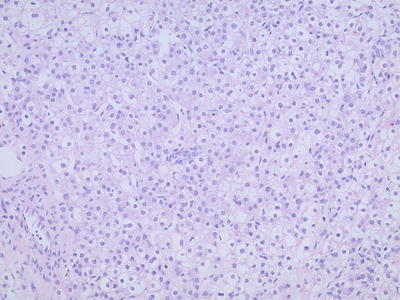
Fig. 8.4
Fetal hepatoblastoma . Well-differentiated neoplastic cells are consistent in size
Table 8.5
Histological classification and characteristics of hepatoblastoma
Epithelial type | Features |
|---|---|
Fetal type | Well differentiated: consistent cellular size (10–20 μm), bright or dark cells, round nucleus, trabecular arrangement, mitotic figures <2/HPF, EMH |
Cell-rich: mitotic figures >2/HPF, obvious nucleolus, litter cytoplasmic glycogen | |
Polymorphic/poorly differentiated | Cellular anaplasia, significant nuclear atypia, increased karyoplasmic ratio, marked nucleoli |
Embryonal | Cellular diameter ranged 10–15 μm, increased karyoplasmic ratio, irregular nucleus, primitive tubular structure, EMH |
Macrotrabecular pattern | Epithelial (fetal or embryonal type) type, trabecular arrangement, intersinusoidal trabecules are thicker than five cells |
Small-cell undifferentiated type | Cellular diameter ranged 5–10 μm, clusters or diffuse distribution, little amphophilic cytoplasm, round or oval nucleus, exquisite chromatin, nonobvious nucleolus, mitotic figures+/−, necrosis, common apoptosis, INI1+/− |
Cholangioblastic type | Tumoral cholangial or tubular structures, often found at the margin of epithelial islets, or as the main component |
Mixed type | |
Without teratomatoid features | Epithelial and mesenchymal components: spindle cells, osteoid, fibrous, chondro |
With teratomatoid features | Epithelium and mesenchyma, primitive nerve epithelium, primitive endoderm, melanoma, squamous and epithelial components |
- 2.
Crowded fetal type (CF). Also known as mitotically active fetal type, the cells of the tumor are morphologically similar to WDF but with more cellular density, clear boundaries, increased karyoplasmic ratio, visible nucleolus, and increased mitotic figures >2个/10HPF (Fig. 8.5). This type is often found in a mixture with WDF, and these tumors with defined CF regions should be treated with postsurgical adjuvant chemotherapy. Glypican-3 staining is beneficial in the differential diagnosis of the two types.
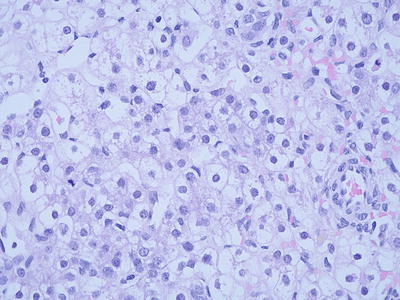
Fig. 8.5
Crowded fetal type hepatoblastoma . Tumor cellular density is increased and the cells have clear boundaries
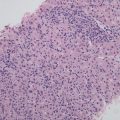
- 3.
Embryonal type. This type of tumors is morphologically similar to the cells in the embryonic liver tissue during sixth to eighth weeks of gestation, which are 10–15 μm in diameter, round or angular, scant cytoplasm, increased karyoplasmic ratio, a little eosinophilic cytoplasm, and unclear cellular membrane. They are arranged in flaky and trabecular, acinar, or rosette-shaped clusters (Fig. 8.6) or papilla similar to yolk sac tumor (Fig. 8.7). Visible primitive tubular structures are formed with accidental tumor cells in the loose myxoid background distributed in microcystic arrangement; tumor cells are densely distributed with obvious nuclear overlap. Extramedullary hematopoiesis (EMH) can also be observed (Fig. 8.8).
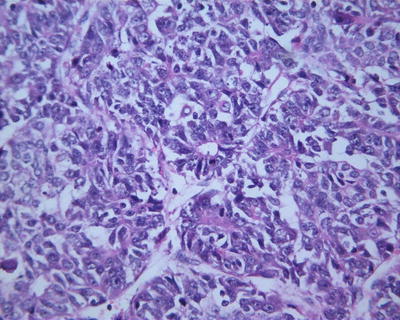
Fig. 8.6
Embryona type hepatoblastoma . The tumor cells are arranged in rosettes shaped clusters with active nuclear mitotic figures
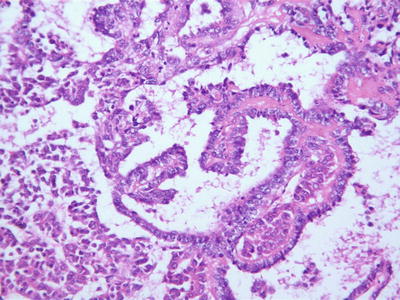
Fig. 8.7
Embryona type hepatoblastoma . The tumor cells are arranged in papillary glandular structure
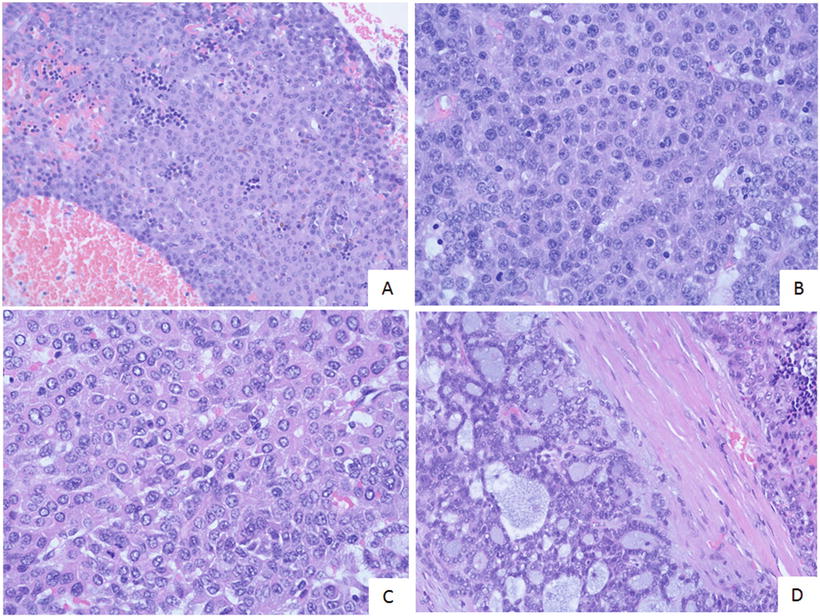
Fig. 8.8
Embryona type hepatoblastoma . (a) Tumor cells are high-density with overlapping nuclear; (b) Tumor cells has scant cytoplasm and the higher karyoplasmic ratio with unclear cellular memberane; (c) Tumor cells are round or angular with eosinophilic cytoplasm and visible nucleoli; (d) Tumor cells are arranged in glandular or micro-cystic shape
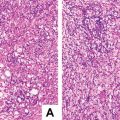
- 4.
Pleomorphic/poorly differentiated. Previously known as “anaplastic fetal type” and “hepatocellular carcinoma type,” it is a rare type of HB , seen in post-chemotherapy metastatic specimens, the morphology of which is similar to pleomorphic tumor cells containing rich eosinophilic cytoplasm in fetal or embryonal HB , increased karyoplasmic ratio, obvious nuclear atypia, thick chromatin, conspicuous nucleolus, visible mitotic figures, or even anaplastic features, including large cells (3–4 times the size of the adjacent cells) and pathological mitotic figures (Fig. 8.9). If these pleomorphic tumor cells grow in huge trabecular pattern, it is difficult to differentiate it from hepatocellular carcinoma , and typical HB regions found in the tumor may facilitate the diagnosis. Rare cases contain simultaneously the components of HB and HCC; however, the relationship between the prognosis and the type of tumor is still not determined.
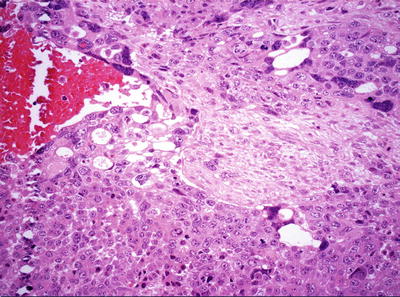
Fig. 8.9
Pleomorphic hepatoblastoma . Pleomorphic and anaplastic tumor giant cells with abundant eosinophilic cytoplasm
- 5.
Get Clinical Tree app for offline access
Cholangioblastic type. This type of HB is rare with some tumor cells undergoing cholangiolar differentiation [9], with or without cholangiolar structures which can be located inside or surrounding the tumor. The cholangioblasts are often cubic rather than columnar, with round nuclei and thick chromatin (Fig. 8.10), which express cholangioepithelial immunophenotypes (CK7, CK19 positive).
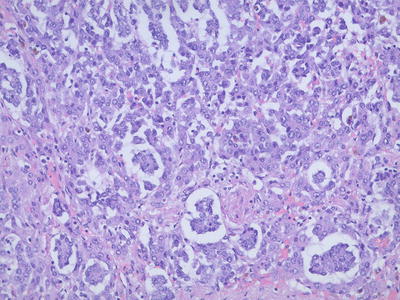
Fig. 8.10
Cholangioblastic type hepatoblastoma , tumor cells undergone cholangio-differentiation with focal cholangiolar structures can be seen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree