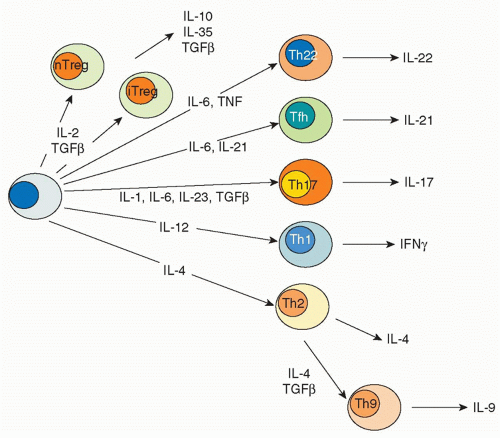
transcription factor responsible for the distinctive pattern of gene expression.21,22 Recently, though, newer fates for helper T cells have been recognized, and they are designated based on expression of their eponymous, signature cytokines: Th17, Th22, and Th9 cells.23 These newly appreciated subsets can also express characteristic, lineage-defining transcription factors; however, as will be discussed, the expression of these master regulators is now recognized to be more promiscuous than previously appreciated. In addition to Th1, Th2, Th17, Th9, and Th22 cells subsets are T cells that reside in proximity to B cells in germinal centers, so-called follicular helper T (Tfh) cells. However, the distinction between Tfh cells and cytokine-secreting effector subsets is also a topic of intense investigation. Finally, there are various types of regulatory T cells. Each subset will be briefly discussed with respect to their characteristics features, inductive factors, key transcription factors, and negative regulators.
TABLE 29.1 Th Subsets | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
and IgG3 isotypes in mice, both of which are stimulated by IFN-γ. IL-4, together with IL-13, promotes the formation of “alternatively activated” macrophages, suppresses IFN-γ-mediated classical macrophage activation and thus inhibits defense against intracellular microbes. IL-4, IL-13, and IL-5 mobilize eosinophils, basophils, and mast cells, and IL-13 increases mucus secretion from airway and gut epithelial cells. These actions contribute to elimination of microbes at epithelial surfaces. In this manner, Th2 cells are important for host defense against helminths and other parasites, but are also the major drivers of allergy and asthma.
signaling also promotes Th17 differentiation.132 As indicated previously, mTor signaling via mTorc1 also promotes Th17 differentiation.45
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree