Heart Failure: Introduction
Heart failure may be defined as an inability of the heart to pump sufficient blood to meet the metabolic needs of the body’s tissues or the ability to do so only at the expense of elevated intracardiac pressures. Heart failure represents a clinical syndrome rather than a specific diagnosis, and, to a large extent, it is a geriatric syndrome in much the same way that dementia and incontinence are geriatric syndromes. Indeed, heart failure may be viewed as the quintessential disorder of cardiovascular aging since, as discussed later in this chapter, extensive age-related changes in cardiovascular structure and function, in conjunction with the rising prevalence of cardiovascular diseases with advancing age and the recent decline in premature cardiovascular deaths, all contribute to an exponential rise in the prevalence of heart failure with advancing age. Thus, although the clinical syndrome of heart failure has been recognized by physicians for more than 2000 years, it has only been within the past two decades that it has been identified as a major public health concern, a development that is largely attributable to the aging of the population.
Epidemiology and Economic Impact
Despite progressive declines in age-adjusted mortality rates from coronary heart disease and hypertensive cardiovascular disease, both the incidence and the prevalence of heart failure are increasing, and it is projected that these trends will continue for the next several decades. As shown in Table 78-1, several factors have contributed to the progressive rise in heart failure. Foremost among these is the increasing number of older adults who, by virtue of advanced age and the high prevalence of hypertension, coronary heart disease, and cardiac valvular disorders in older individuals, are predisposed to the development of heart failure. In addition, advances in the treatment of other acute and chronic cardiac and noncardiac conditions, most notably atherosclerotic heart disease, hypertension, renal failure, cancer, and infectious diseases, have paradoxically contributed to the increasing burden of heart failure. Thus, individuals who 20 years ago might have died in middle age from acute myocardial infarction are now surviving to older age only to develop heart failure in their later years. Similarly, improved blood pressure control has led to a 60% decline in stroke mortality over the last 30 years, yet these same patients remain at risk of the subsequent development of heart failure as a complication of hypertension and left ventricular hypertrophy.
Aging of the population
|
Improved therapy for coronary heart disease and hypertension
|
Improved therapy for other disorders
|
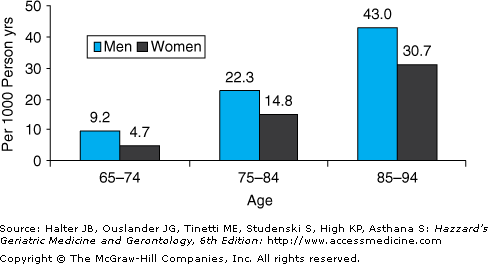
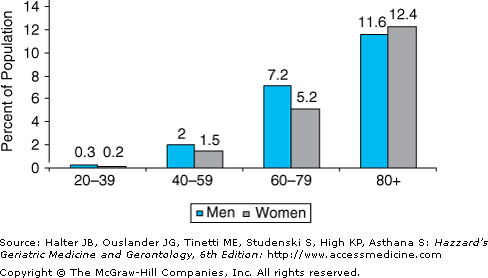
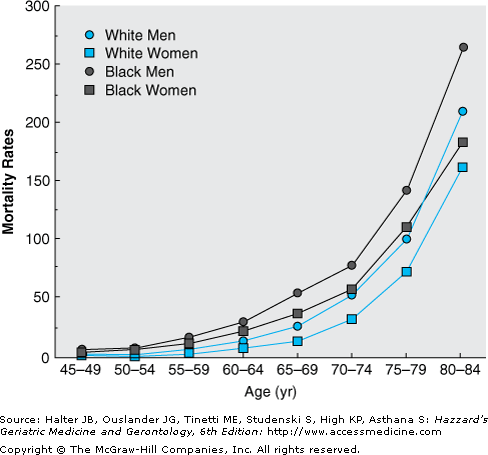
Heart failure affects approximately 5.2 million Americans, and 550 000 new cases are diagnosed each year. Moreover, both the incidence and the prevalence of heart failure are strikingly age dependent (Figures 78-1 and 78-2). Thus, heart failure is relatively uncommon in individuals younger than 40 years, but the prevalence doubles for each decade thereafter and exceeds 10% in adults older than 80 years. Similarly, heart failure mortality rates increase exponentially with advancing age in all major demographic subgroups of the U.S. population (Figure 78-3).
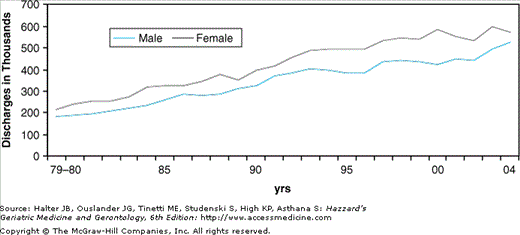
Heart failure is also a major source of chronic disability and impaired quality of life in older adults, and it is currently the leading indication for hospitalization in individuals older than 65 years. Moreover, the number of hospital discharges for heart failure increased by more than twofold from 1979 to 2004, primarily owing to the aging of the population (Figure 78-4). In 2004, there were 1.1 million hospital admissions in the United States with a primary diagnosis of heart failure (Table 78-2). Of these, 75% were in patients older than 65 years and more than 50% occurred in patients 75 years of age or older. The majority of heart failure patients younger than 65 years are males, but women comprise nearly 60% of heart failure admissions after the age of 65 years, and the proportion of females continues to rise with advancing age. The prevalence of heart failure in older Caucasians and African-Americans is similar, but hospital admission rates are lower in Hispanics and Asians. Whether this represents a true difference in population prevalence or a cultural difference in the likelihood that affected individuals will seek medical attention is unknown. Heart failure is also a common reason for outpatient physician office visits, with estimates ranging from 3 to 12 million office visits annually. In this regard, heart failure ranks second only to hypertension among cardiovascular causes for outpatient physician visits.
POPULATION GROUP | PREVALENCE* | INCIDENCE | MORTALITY* | HOSPITAL DISCHARGES | COST† |
|---|---|---|---|---|---|
Both sexes | 5.2 million (2.5%) | 550 000 | 57 700 | 1.099 million | $33.2 |
Men | 2.6 million (2.8%) | 22 501 (39%) | 524 000 | ||
Women | 2.6 million (2.2%) | 35 199 (61%) | 575 000 | ||
Race/ethnicity | |||||
White men | 2.8% | 20 040 | |||
White women | 2.1% | 31 785 | |||
Black men | 2.7% | 2119 | |||
Black women | 3.3% | 3017 | |||
MA men | 2.1% | NA | |||
MA women | 1.9% | NA |
As a result of its high prevalence and the need for intensive resource use in both the inpatient and the outpatient settings, it is not surprising that the economic burden of heart failure is staggering. Heart failure is currently the most costly diagnosis-related group in the United States, with estimated total annual expenditures in excess of $33 billion. Indeed, inpatient expenditures for heart failure exceed those for all cancers combined by a factor of 2.4 and for all myocardial infarctions combined by a factor of 1.7.
Pathophysiology
Heart failure represents the prototypical disorder of cardiovascular aging in that age-related changes in the cardiovascular system in concert with an increasing prevalence of cardiovascular diseases at older age conspire to produce an exponential rise in heart failure prevalence with advancing age.
Aging is associated with extensive changes in cardiovascular structure and function (see Chapter 74). However, in the absence of coexistent cardiovascular disease, resting cardiac function is well preserved even at very elderly age. Thus, the resting left ventricular ejection fraction, an index of left ventricular systolic performance, is unaffected by age in healthy individuals. Similarly, most studies indicate that resting cardiac output is either maintained or declines minimally with normal aging.
From the clinical perspective, the changes associated with cardiovascular aging result in an impaired ability of the heart to respond to stress, whether that stress is physiologic (e.g., exercise) or pathologic (e.g., hypertension or myocardial ischemia). Four principal changes in the cardiovascular system contribute directly to the heart’s attenuated capacity to augment cardiac output in response to stress. First, aging is associated with reduced responsiveness to β-adrenergic stimulation. The mechanism underlying this change has not been fully elucidated, but it is not caused by reduced circulating catecholamine levels, decreased β-receptor density on cardiac myocytes, or altered responsiveness to intracellular calcium. In any case, the diminished response to β-adrenergic stimulation limits the heart’s capacity to maximally increase heart rate and contractility in response to stress, and β2-mediated peripheral vasodilatation is also compromised.
A second major effect of aging is increased vascular stiffness, primarily because of increased collagen deposition and cross-linking and degeneration of elastin fibers in the media and adventitia of the large- and medium-sized arteries. Increased vascular stiffness results in increased impedance to left ventricular ejection (i.e., increased afterload), and it also contributes to the increased propensity of older individuals to develop isolated systolic hypertension.
A third major effect of aging is altered left ventricular diastolic filling. Diastole is characterized by four phases: isovolumic relaxation, early rapid filling, passive filling during mid-diastole, and late filling owing to atrial systole. The first two phases, isovolumic relaxation and early rapid filling, are largely dependent on myocardial relaxation, an active, energy-requiring process, whereas filling during the latter two phases is governed principally by intrinsic myocardial “stiffness,” or compliance. Aging is associated with impaired calcium release from the contractile proteins and reuptake by the sarcoplasmic reticulum at the end of systole, leaving the heart in a state of “partial contraction” at the onset of diastole and inhibiting early diastolic relaxation. In addition, increased interstitial connective tissue content and collagen cross-linking reduce ventricular compliance. Compensatory myocyte hypertrophy in response to increased ventricular afterload and myocyte loss because of apoptosis further compromise left ventricular compliance. Thus, normal aging is associated with important changes that impair both relaxation and compliance, adversely impacting all four phases of diastole and substantially altering the pattern of left ventricular diastolic filling.
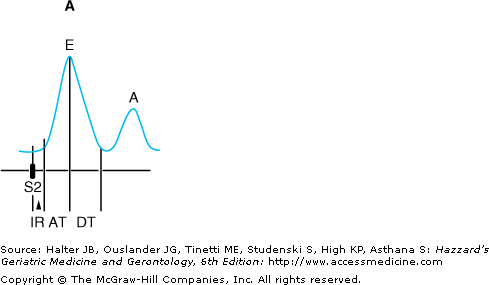
Age-related changes in diastolic filling and atrial function can be evaluated noninvasively using Doppler echocardiographic techniques to examine diastolic inflow across the mitral valve (Figure 78-5). In healthy young persons, the transmitral inflow pattern is characterized by a large E-wave, with a rapid upstroke representing rapid filling of the ventricle immediately following the opening of the mitral valve and corresponding to active ventricular relaxation (Figure 78-5A). This is followed by a period in which the rate of filling slows (the downslope of the E-wave), mid-diastolic diastasis (in which left atrial and left ventricular pressures are essentially equal), and a second burst of flow at the end of diastole corresponding to atrial contraction (the A-wave, or atrial “kick”). Importantly, the majority of ventricular filling occurs in the first half of diastole in young individuals, with a relatively small contribution from atrial contraction.
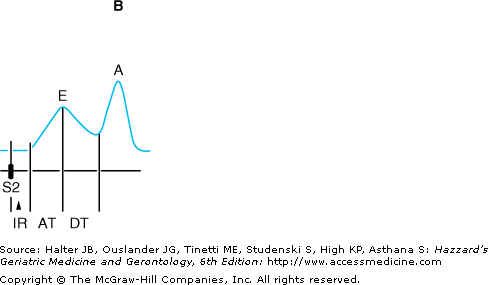
Figure 78-5.
Schematic diagram of Doppler echocardiographic mitral valve inflow patterns. (A) Normal pattern. (B) Impaired filling pattern. (C) Restrictive pattern. AT, acceleration time; DT, deceleration time; IR, isovolumic relaxation; S2 aortic valve closure. (Adapted with permission from Feigenbaum H. Echocardiography. 5th ed. Philadelphia: Lea & Febiger; 1994:152.)
In older persons, alterations in cardiac relaxation and compliance result in characteristic changes in the pattern of diastolic filling (Figure 78-5B). Early filling is impaired, and the upstroke of the E-wave is delayed. Similarly, the downslope of the E-wave is less steep, as it takes a longer time to achieve diastasis. In order to compensate for increased resistance to emptying, the left atrium enlarges and hypertrophies. This results in a more forceful left atrial contraction and an augmented A-wave. As a result of these changes, a greater proportion of filling occurs in the second half of diastole in older individuals, and as much as 30% to 40% of left ventricular end-diastolic volume may be attributable to atrial contraction. Thus, older individuals become increasingly reliant on the atrial “kick” to maximize left ventricular filling.
A third pattern of diastolic filling, referred to as the restrictive pattern, occurs when the left ventricle’s ability to accept blood becomes severely compromised. In this situation (Figure 78-5C), very little flow occurs after the rapid filling phase in early diastole. This pattern is characterized by a tall, narrow E-wave with a rapid downslope, as diastasis is achieved early in diastole. Little additional flow occurs during mid-diastole, and the A-wave is typically small, with an amplitude that is less than 50% of the E-wave. A restrictive pattern generally indicates marked elevation of the left ventricular diastolic pressure, and it is generally associated with a poor prognosis, particularly in patients with concomitant systolic dysfunction. However, the restrictive pattern generally occurs in patients with advanced cardiac disease, and it rarely results from aging alone.
Age-related changes in diastolic filling have several important clinical implications. First, inability to distend the cardiac myocytes to an optimal fiber length results in a failure of the Frank–Starling mechanism, one of the cardinal adaptive responses (along with sympathetic activation) for acutely increasing cardiac output. Second, impaired diastolic filling results in a shift to the left in the normal ventricular pressure–volume relationship; i.e., a small increase in diastolic volume is associated with a greater increase in diastolic pressure in older compared to younger individuals. This increase in diastolic pressure is transmitted back to the left atrium, and left atrial myocytes become “stretched.” This, in turn, increases the likelihood of atrial ectopic beats and atrial arrhythmias, especially atrial fibrillation. This accounts, in part, for the fact that atrial fibrillation, like heart failure, increases in prevalence with advancing age. In addition, atrial fibrillation itself is a common precipitant of heart failure in older adults for two reasons. First, the absence of a coordinated atrial contraction substantially compromises late diastolic filling as a result of loss of the atrial “kick.” Second, the rapid, irregular ventricular rate associated with acute atrial fibrillation shortens the diastolic filling period, which further attenuates ventricular filling.
A third effect of altered diastolic filling is an increased propensity for older adults to develop diastolic heart failure (i.e., heart failure with normal left ventricular systolic function). Because of the altered left ventricular pressure–volume relation, increases in left ventricular pressure owing to ischemia or uncontrolled hypertension may lead to pulmonary congestion and edema. Moreover, individuals with impaired diastolic function are often “volume sensitive”; i.e., small increments in intravascular volume, as may occur with a dietary salt load or intravenous fluid administration, are poorly accommodated by the noncompliant ventricle. As a result, intraventricular pressure rises abruptly and heart failure ensues. Conversely, intravascular volume contraction, which may arise from poor oral intake or overdiuresis, can cause a marked fall in intraventricular volume, which in turn leads to a fall in stroke volume and cardiac output.
The fourth major effect of cardiovascular aging is altered myocardial energy metabolism at the level of the mitochondria. Under resting conditions, older cardiac mitochondria are able to generate sufficient quantities of adenosine triphosphate to meet the heart’s energy requirements. However, when stress causes an increase in adenosine triphosphate demands, the mitochondria are often unable to respond appropriately. Although the precise mechanism underlying this mitochondrial failure is unclear, the defect adds to the heart’s inability to maintain normal metabolic function under stress.
To summarize, four major age-related changes in cardiovascular structure, function, and physiology combine to reduce cardiovascular reserve and greatly increase the risk of heart failure in older adults. Recalling that cardiac output is determined by four primary factors (heart rate, preload, afterload, and contractile state), and recognizing that each of these factors is adversely affected by one or more of the four major effects of aging on the heart, and that superimposed upon these changes is the high prevalence of cardiac disease in older adults, it is indeed not surprising that the incidence and prevalence of heart failure rise exponentially with advancing age.
It is also important to note that aging is associated with significant changes in other organ systems, which impact directly or indirectly on the development and/or management of heart failure. Aging is accompanied by a decline in glomerular filtration rate, and the aging kidney is less able to maintain intravascular volume and electrolyte homeostasis (see Chapter 85). The reduced capacity of the kidneys to respond to intravascular volume overload or dietary sodium excess further increases the risk of heart failure in older individuals. In addition, older patients are less responsive to diuretics and more likely to develop diuretic-induced electrolyte abnormalities than younger patients, factors that may complicate the management of heart failure in the older age group.
Aging is also associated with numerous changes in respiratory function, which serve to diminish respiratory reserve (see Chapter 82). Some of these effects, such as V/Q mismatching and sleep-related breathing disorders, may contribute directly to the development of heart failure by producing hypoxemia or pulmonary hypertension. Other changes reduce the capacity of the lungs to compensate for the failing heart by increasing tidal volume and minute ventilation, thereby contributing to the patient’s sensation of dyspnea. In more severe cases of cardiac failure, such as pulmonary edema, acute respiratory failure may ensue, partly as a consequence of the inability of the lungs to maintain oxygenation and effective ventilation.
Age-related changes in nervous system function include an impaired thirst mechanism, which may contribute to dehydration and intravascular volume contraction in patients treated with diuretics, and reduced capacity of the central nervous system’s autoregulatory mechanisms to maintain cerebral perfusion in the face of changes in systemic arterial blood pressure. The latter effect may contribute to subtle changes in mental function in older heart failure patients treated with vasodilators. Aging is also associated with widespread changes in reflex responsiveness. For example, impaired responsiveness of the carotid baroreceptors to acute changes in blood pressure may cause orthostatic hypotension or syncope, and these effects may further be aggravated by many of the drugs used to treat heart failure.
Finally, as is well recognized, aging is associated with significant changes in the pharmacokinetics and pharmacodynamics of almost all drugs. In addition, older patients tend to be at increased risk of both drug–drug and drug–disease interactions as a result of the high prevalence of comorbid conditions and the use of multiple pharmacological agents. These factors often lead to alterations in drug efficacy and an increased side-effect profile, and these effects must be taken into consideration when designing therapy for older heart failure patients (see Chapters 8 and 24).
Etiology and Precipitating Factors
In general, the etiology of heart failure is similar in older and younger patients (Table 78-3), but heart failure in older individuals is more often multifactorial. As in younger patients, hypertension and coronary heart disease are the most common causes of heart failure, accounting for more than 70% of cases. Hypertensive hypertrophic cardiomyopathy represents a more severe form of hypertensive heart disease most commonly seen in older women and often accompanied by calcification of the mitral valve annulus. These patients often manifest severe diastolic dysfunction and may exhibit dynamic left ventricular outflow tract obstruction indistinguishable from that seen in classical hypertrophic cardiomyopathy.
Coronary artery disease
|
Hypertensive heart disease
|
Valvular heart disease
|
Cardiomyopathy
|
Infective endocarditis |
Myocarditis |
Pericardial disease |
High-output failure
|
Age-related diastolic dysfunction |
Valvular heart disease is an increasingly common cause of heart failure at older age. Calcific aortic stenosis is now the most common form of valvular heart disease requiring surgical intervention, and aortic valve replacement is the second most common open heart procedure performed in patients older than 70 years (after coronary bypass grafting). Mitral regurgitation in older individuals may be caused by myxomatous degeneration of the mitral valve leaflets and chordae tendineae (mitral valve prolapse), mitral annular calcification, valvular vegetations, ischemic papillary muscle dysfunction, or altered ventricular geometry owing to ischemic or nonischemic dilated cardiomyopathy. Importantly, mitral regurgitation may be acute (e.g., following acute myocardial infarction), subacute (e.g., endocarditis), or chronic (e.g., myxomatous degeneration), and the clinical manifestations may vary widely in each of these settings. In the United States, rheumatic mitral stenosis is a less common cause of heart failure in older adults, but it is still occasionally seen. Functional mitral stenosis owing to severe mitral valve annulus calcification with encroachment on the mitral valve orifice is an uncommon cause of heart failure, but it is associated with a poor prognosis. Aortic insufficiency may be either acute (e.g., because of endocarditis or type A aortic dissection) or chronic (e.g., annuloaortic ectasia or syphilitic aortitis), but it is a relatively infrequent cause of heart failure in older adults. Finally, prosthetic valve dysfunction should be considered as a potential cause of heart failure in any patient who has undergone previous valve repair or replacement.
Cardiomyopathies are classified into three categories: dilated, hypertrophic, and restrictive. In older adults, ischemic heart disease with one or more prior myocardial infarctions is the most common cause of dilated cardiomyopathy. Nonischemic dilated cardiomyopathy is less common in older than in younger individuals; when present, it is most often either idiopathic in origin or attributable to chronic ethanol abuse. Less frequently, dilated cardiomyopathy may be caused by cancer chemotherapy (e.g., anthracyclines and trastuzumab) or other causes. Classical hypertrophic cardiomyopathy, once thought to be rare in the geriatric age group, has been increasingly recognized in older adults since the advent of echocardiography. Similarly, restrictive cardiomyopathy, most commonly owing to amyloid deposition (so-called senile cardiac amyloid), is an occasional cause of heart failure. In one autopsy series, cardiac amyloid deposition was thought to be clinically important in approximately 10% of individuals 90 years of age or older.
Infective endocarditis is an uncommon but important cause of heart failure in older patients because it is one of the few etiologies for which curative pharmacological therapy is available. Endocarditis should be strongly suspected in any patient with persistent fever and either a prosthetic heart valve or a preexisting valvular lesion. It should also be considered in any patient with fever, recent dental work or other procedure, and a new or worsening heart murmur. It is important to recognize, however, that the clinical manifestations of endocarditis are often protean, and the absence of fever or a heart murmur does not exclude this diagnosis in older individuals.
Myocarditis is a relatively rare cause of heart failure in older adults. It may be infectious (e.g., postviral) or noninfectious (e.g., owing to sarcoid or collagen vascular disease). Pericardial effusions, for which there are numerous etiologies, occasionally present with heart failure symptomatology, including fatigue, exertional dyspnea, and edema. Constrictive pericarditis may be infectious (e.g., tuberculous) or noninfectious (e.g., postradiation), but it is a rare cause of heart failure in older patients.
High-output failure is an uncommon cause of heart failure in older adults, but when present the diagnosis is frequently overlooked. Potential causes of high-output failure include chronic anemia, hyperthyroidism, thiamine deficiency, and arteriovenous shunting (e.g., owing to a dialysis fistula or arteriovenous malformations).
Finally, in a small percentage of older heart failure patients, detailed investigation may fail to identify any primary cardiovascular pathology. In cases with normal left ventricular systolic function, heart failure may be attributed to age-related diastolic dysfunction.
In addition to determining the etiology of heart failure, it is important to identify coexisting factors that may have contributed to the acute or subacute exacerbation (Table 78-4). The most common precipitant in patients with preexisting heart failure is noncompliance with medications and/or diet. Indeed, noncompliance may contribute to as many as two-thirds of heart failure exacerbations. In hospitalized patients, iatrogenic volume overload is also an important precipitant of heart failure.
Myocardial ischemia or infarction |
Dietary sodium excess |
Excess fluid intake |
Medication noncompliance |
Iatrogenic volume overload |
Arrhythmias |
|
Associated medical conditions
|
Drugs and medications
|
Among cardiac factors, myocardial ischemia or infarction and new-onset atrial fibrillation or flutter are the most common causes of an acute episode of heart failure. Other cardiac causes include ventricular arrhythmias, especially ventricular tachycardia, and bradyarrhythmias, such as marked sinus bradycardia or advanced atrioventricular block. Sick sinus syndrome, which is common in older adults, is a frequent cause of bradyarrhythmias in this population.
As previously discussed, older patients have limited cardiovascular reserve and they are less able to compensate in response to increased demands. As a result, heart failure in older adults is often precipitated by acute or worsening noncardiac conditions. Patients with acute respiratory disorders, such as pneumonia, pulmonary embolism, or an exacerbation of chronic obstructive lung disease, are particularly prone to exhibit deterioration in cardiac function. Other serious infections, such as sepsis or pyelonephritis, may also lead to heart failure exacerbations. In patients with hypertension, inadequate blood pressure control is a common cause of worsening heart failure. Thyroid disease, anemia (e.g., owing to gastrointestinal bleeding), and declining renal function may also contribute directly or indirectly to the development of heart failure.
Finally, numerous drugs and medications may contribute to heart failure exacerbations. Alcohol is a cardiac depressant, and it may also precipitate arrhythmias, especially atrial fibrillation. Beta-blockers (including ophthalmologic agents) and calcium antagonists are widely used in older individuals with cardiovascular disease, but both classes of agents are negatively inotropic and may exacerbate heart failure. Class Ia (e.g., quinidine, procainamide, and disopyramide) and Ic (e.g., flecainide and propafenone) antiarrhythmic agents have important myocardial depressant effects that may worsen cardiac function. Nonsteroidal anti-inflammatory drugs (NSAIDs), which are widely used by older adults, impair renal sodium and water excretion and may, therefore, contribute to intravascular volume overload. In addition, NSAIDs antagonize the effects of angiotensin-converting enzyme (ACE) inhibitors, thereby limiting the efficacy of these agents. Corticosteroids and estrogen preparations may cause fluid retention and an increase in total body water. Fluid retention is the most important side effect of the insulin-sensitizing thiazolidinediones (rosiglitazone and pioglitazone), and worsening heart failure may occur with these agents. The antihypertensive agent minoxodil also promotes fluid retention, and several other antihypertensive drugs (e.g., clonidine and guanethidine) may have unfavorable hemodynamic effects.
Clinical Features
As in younger patients, the most common symptoms of heart failure in older adults are exertional shortness of breath, orthopnea, dependent edema, fatigue, and exercise intolerance. However, there is an increased prevalence of atypical symptomatology in older patients, particularly those older than 80 years (Table 78-5). As a result, heart failure in older adults is paradoxically both over- and underdiagnosed. Thus, shortness of breath and orthopnea in an older individual may be attributed to heart failure when the underlying cause is chronic lung disease, pneumonia, or pulmonary embolism. Similarly, fatigue and reduced exercise tolerance may be caused by anemia, hypothyroidism, depression, or poor physical conditioning. On the other hand, sedentary individuals and those limited by arthritis or neuromuscular conditions may not report exertional dyspnea or fatigue, and atypical symptoms such as those listed in Table 78-5 may be the first and only clinical manifestations of heart failure. In such cases, the physician must maintain a high index of suspicion, or the diagnosis of heart failure may be readily overlooked.
Nonspecific systemic complaints
|
Neurological symptoms
|
Gastrointestinal disorders
|
As with symptoms, the physical findings in older heart failure patients may be nonspecific or atypical. The classic signs of heart failure include moist pulmonary rales, an elevated jugular venous pressure, abdominojugular reflux, an S3 gallop, and pitting edema of the lower extremities. However, pulmonary rales in older individuals may be due to chronic lung disease, pneumonia, or atelectasis, and peripheral edema may be caused by venous insufficiency, renal disease, or medication (e.g., calcium channel-blockers). Conversely, older patients may have an essentially normal physical examination despite markedly reduced cardiac performance. Alternatively, impaired sensorium or Cheyne–Stokes respirations may be the only findings to suggest the presence of heart failure.
The clinical manifestations of systolic and diastolic heart failure are similar, and no single clinical feature can reliably distinguish heart failure patients with intact left ventricular systolic function from those with impaired contractility. Nonetheless, certain features tend to favor one form or the other (Table 78-6). Based on the presence or absence of specific features, the probability of normal or reduced systolic function can be estimated, and there have been several attempts to develop algorithms for distinguishing these syndromes. Unfortunately, the predictive accuracy of these algorithms has been modest, and additional testing is essential in order to reliably differentiate systolic from diastolic heart failure.
Systolic Dysfunction | Diastolic Dysfunction | |
|---|---|---|
Demographics | Age <60 yr | Age >70 yr |
Male gender | Female gender | |
Comorbid illnesses | Prior myocardial infarction | Chronic hypertension |
Alcoholism | Renal disease | |
Valvular insufficiency | Obesity | |
Aortic stenosis | ||
Presentation | Progressive shortness of breath | Acute pulmonary edema |
Atrial fibrillation | ||
Physical examination | Normotensive or hypotensive | Hypertensive |
Jugular venous distention | Absence of jugular venous distention | |
Displaced PMI | Sustained PMI | |
S3 gallop | S4 gallop | |
Pitting edema | Absence of peripheral edema | |
Electrocardiogram | Q-waves and prior myocardial infarction | Left ventricular hypertrophy |
Chest x-ray | Marked cardiomegaly | Normal or mildly increased heart size |
Diagnostic Evaluation
Heart failure may be difficult to diagnose in older patients with multiple comorbid conditions and either vague or nonspecific symptoms and signs. Thus, the first task facing the physician is to establish whether or not heart failure is present. This begins with a careful history and physical examination, giving due consideration to potential alternative etiologies for the patient’s findings. As discussed in the previous section, physical signs may be unreliable in older patients. Nonetheless, certain findings, including pulsus alternans, an S3 gallop, and the presence of jugular venous distension at rest or in response to the abdominojugular reflux maneuver, are highly specific signs of heart failure in older patients. In the absence of these findings, the diagnosis often remains in doubt, and additional laboratory studies are required.