The net state of immunosuppression is a complex function determined by the interaction of a number of factors.
SOLID ORGAN TRANSPLANT
Numerous factors affect the net degree of immunosuppression. First, underlying disease processes, such as diabetes or systemic lupus erythematosus, may themselves compromise the inflammatory response. Second, Immunosuppressive medications are given routinely to control several conditions that may
come to transplant such as chronic hepatitis, biliary cirrhosis, or inflammatory lung disease. The intensity and duration of such immunosuppressive therapy administered has a direct impact on the net state of immune compromise. Third, active infection with one or more immunomodulating viruses (e.g., cytomegalovirus [CMV], Epstein—Barr virus [EBV], human herpesvirus-6, hepatitis viruses B [HBV] or C [HCV], or HIV) may add to the net state of suppression. Finally, conditions common to the immunocompromised host such as damage to the mucocutaneous surfaces of the body, neutropenia, or metabolic abnormalities such as protein—calorie malnutrition, uremia, or hyperglycemia all may additionally compromise immune function.
These numerous potential contributors to the net state of immunosuppression may be dwarfed by the potent immunosuppression given to prevent graft rejection. The standard of care for SOT recipients is a multidrug regimen consisting of a calcineurin inhibitor (cyclosporine or tacrolimus), prednisone, and either azathioprine or mycophenolate. In addition, antithymocyte globulin (ATG; a polyclonal anti—T cell drug) or OKT3 (a monoclonal anti—T cell drug) can be used as induction therapy, especially in kidney and pancreas transplantation (
Table 45.1).
The calcineurin inhibitors are the cornerstones of modern antirejection therapy. They exert their effects through a complex signaling pathway that results in the inhibition of the transcriptional activation of genes required for T-cell activation, proliferation, and function. This results in the inhibition of a large number of proinflammatory cytokines, with the most important of these effects being the blockade of essential functions of IL-2. The infectious disease consequences of these drugs are direct results of these mechanisms: a dose-related inhibition of microbial-specific T-cell cytotoxic activity, thus promoting numerous infections, including those caused by the herpes group viruses, various fungi, mycobacteria, and the many intracellular infections. The key toxicities of the calcineurin inhibitors are injury to the kidneys and hypertension (
48,
49).
HEMATOPOIETIC STEM CELL TRANSPLANT
The factors similar to those outlined for SOT affect the risk of infection for HSCT. The nature and intensity of the immunosuppressive regimen used before transplant as treatment of the underlying cancer is a major determinant of infections occurring early on. Examples include the use of agents such as purine analogs, alemtuzumab, pentostatin, and rituximab, which are associated with immunosuppressive effects lasting from months to years.
However, additional factors unique to the HSCT population add considerably to the net state of immunosuppression. Additional immunosuppression will occur at the time of conditioning when the patient receives total body irradiation or ATG. Furthermore, the stem cell source (e.g., cord blood grafts can take longer to engraft, increasing the infection risk in the early posttransplant period), graft manipulation such as T-cell depletion (reduces passively transferred T-cell immunity, especially against viruses, and impairs T-cell reconstitution after transplant), and donor matching (determines the risk of GVHD) influence the risk for infection. After transplant, the
occurrence of GVHD and its treatment with pharmacologic immunosuppression, as well as the immunomodulating infections listed above determine the pace of immune reconstitution and susceptibility to infection.
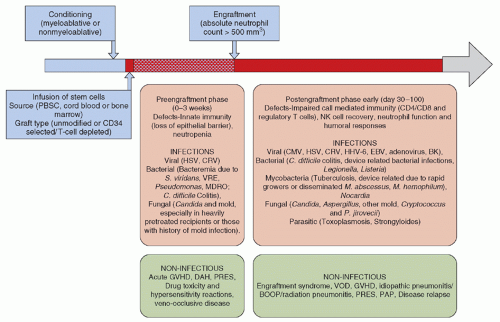
The course of allogeneic HSCT is shown in
Figure 45.3 and consists of conditioning therapy (myeloablative or nonmyeloablative) followed by infusion of stem cells, which are mostly derived from peripheral or cord blood. The graft may be manipulated for CD34 selection or T-cell depletion. Engraftment usually occurs between 2 and 3 weeks, depending upon the transplant type and source of stem cells. The time to engraftment or marrow reconstitution is typically longer for CBT as cord blood contains fewer nucleated cells despite the use of double cord units (DUCBT). Furthermore, these cells necessarily are immunologically naive, further delaying immune recovery. In some studies, the prolonged period of neutropenia and lymphopenia after CBT has been associated with a higher risk of infectious complications in the first 100 days after CBT without any substantial impact on nonrelapse mortality (
50,
51,
52,
53,
54,
55).
T-cell depletion reduces the risk of GVHD by eliminating donor T cells, but increases the risk of PTLD (posttransplant lymphoproliferative disorder) and infection, especially invasive fungal infections and severe viral diseases (
56,
57,
58). Most techniques used for T-cell depletion are ex vivo by physical separation or immunologic manipulation of the graft with the use of monoclonal antibodies (anti-CD2, anti-CD3, anti-CD5, anti-CD52, anti-CD25, and anti-CD8). In vivo T-cell depletion is attained with the use of antilymphocyte antibodies (ATG or alemtuzumab) in conditioning regimens.
T-cell depletion is a strategy used to reduce the incidence of GVHD after transplant from HLA nonidentical donors. Prophylaxis for GVHD is routinely used in all other forms of allogeneic transplant. Although graft vs. leukemia (GVL) effect would diminish with the routine use of prophylaxis, the use of posttransplant donor lymphocyte infusions (DLI) allows achievement of the desired immunologic state despite anti-GVHD prophylaxis. Cyclosporine and methotrexate (with or without corticosteroids) or tacrolimus with methotrexate is the most commonly used regimen for prevention of acute GVHD (
59,
60). Alternate prophylactic agents, in the event of significant toxicity (especially nephrotoxicity with cyclosporine and tacrolimus), include sirolimus and mycophenolate. In the absence of GVHD, prophylaxis is generally continued for 6 months for identical donors and longer in mismatched transplants.
Corticosteroids (methylprednisolone) are the mainstay for the management of acute GVHD, and more intense immunosuppressive regimens (e.g., cyclosporine, tacrolimus, infliximab, daclizumab, alemtuzumab) are reserved for steroid refractory patients. The mechanism of action of drugs used in the prevention and management of acute GVHD, common adverse effects, and infectious complications are discussed below.
EFFECTS OF PHARMACOLOGIC IMMUNOSUPPRESSION ON THE OCCURRENCE OF INFECTION
The major determinants of the net state of immunosuppression are the dose, duration, and temporal sequence in which the immunosuppressive regimen is administered. A brief description of some of the key agents and novel immunomodulating drugs used in SOT and HSCT that are particularly associated with an increased risk of HAI and other serious infections is provided here. Because of these agents, the range of HAIs in this population is distinct from those found on a general ward, so approaches to surveillance should be tailored accordingly. In addition, they may predispose to inflammatory disorders, such as interstitial pneumonitis that may appear to be an HAI, such as ventilator-associated pneumonia (VAP), thereby complicating surveillance.
Purine Analogs
Purine analogs are structurally similar to purine metabolites and inhibit DNA synthesis and repair. The most widely used agents in this class include fludarabine, pentostatin (ADA inhibitor; ADA plays a role in T-cell and B-cell differentiation), and cladribine (ADA-resistant nucleoside analog) (
61,
62). These agents are mostly used in the treatment of lymphoid malignancies. In addition, fludarabine commonly is used in high doses along with busulfan in preparative conditioning regimen for myeloablative HSCT and in lower doses, in combination with busulfan/melphalan with or without low-dose radiation, for nonmyeloablative preparative regimens. Purine analogs induce profound neutropenia, CD4 lymphopenia (lasting ≥1 year for fludarabine and 2 to 4 years after cladribine and pentostatin) (
63), as well as B-cell and monocyte dysfunction. As a result, they increase host susceptibility to a wide range of pathogens including bacterial (encapsulated bacteria,
Listeria spp.,
Legionella spp.,
M. tuberculosis, nontuberculous mycobacteria,
Nocardia spp.), viral (CMV, herpes simplex virus [HSV], VZV),
Pneumocystis jiroveci pneumonia (PCP), and
Cryptococcus spp. Because infection has been reported months after the use of these agents, prophylaxis for PCP is routinely recommended (
64,
65,
66,
67).
Alemtuzumab
Alemtuzumab (Campath) is a humanized monoclonal antibody targeting CD52 (glycoprotein expressed on B-and T lymphocytes, monocytes, and NK cells). Profound depletion of all lymphocyte subsets is observed after alemtuzumab administration and CD4 counts may remain suppressed for up to 2 years (median, 9 months) (
68,
69,
70). It has been used in induction regimen for SOT to prevent acute rejection as well for the treatment of steroid refractory acute rejection (
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81). In HSCT, alemtuzumab has been used in conditioning regimen in allogeneic transplants, especially RIC, to promote engraftment and reduce the risk of GVHD (
82,
83,
84,
85,
86). Alemtuzumab renders the host susceptible to a wide range of pathogens owing to the associated immune defects (
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102).
In one small study of 27 patients receiving treatment with alemtuzumab for lymphoproliferative diseases, Martin et al. (
98) reported opportunistic infections (OIs) in 56% of patients (median time to OI after the alemtuzumab dose was 165 days) and non-OIs occurred in 22/27 (82%) patients. Non-OIs included community respiratory virus infections in 22 patients, device-related infections, bacteremia, or other serious bacterial infections including those due to multidrug-resistant organisms (MDROs) in eight patients each. These findings highlight the impact of immunosuppressive regimen on occurrence and outcome of common HAIs and heighten the need for surveillance and prevention in this population.
Antithymocyte Globulin
ATG is a polyclonal immunoglobulin—horse-or rabbit-derived antibodies against human T cells. ATG is used in conditioning regimens for HSCT to prevent GVHD and as an induction agent in SOT for the prevention of acute graft rejection (
103). The spectrum of infection seen after ATG is similar to alemtuzumab. The use of ATG increases the risk of EBV-PTLD, with the highest risk in conditioning regimens that use ex vivo T-cell depletion along with ATG (71%) (
104,
105,
106,
107). An important complication associated with ATG infusion is the cytokine release “storm” manifesting as high fevers, rigors, and chills, and is often managed by the administration of corticosteroids.
Anti-Interleukin-2Rα Antibodies
The newer agents daclizumab and basiliximab (IL-2Rα receptor antibodies) do not cause cytokine storm and are used in combination with ATG for the prophylaxis of allograft rejection after SOT (mostly renal) and treatment of steroid refractory GVHD (
108,
109). These drugs act by competitive inhibition of IL-2 binding sites on activated T cells and suppression of IL-2-mediated T-cell responses. A number of infections have been associated with the use of daclizumab, including EBV-PTLD, CMV, influenza-like illness (IFI), toxoplasmosis, mycobacterial infections, or severe respiratory viral infection.
However, most of these infections have occurred in the context of heavy prior immunosuppression for the treatment of refractory GVHD (
110,
111,
112,
113). There is insufficient experience with basiliximab, although some studies in renal transplants suggest an overall higher risk of infections (except IFI) with this agent in comparison to alemtuzumab (
102).
Rituximab
Rituximab is a chimeric murine-human monoclonal antibody against CD20 antigen that is expressed on B cells. Primarily used in autologous HSCT in the treatment of B-cell lymphomas, rituximab has a major therapeutic role in the management of PTLD and emerging role in renal transplant in the prevention of graft rejection after ABO incompatible match, B cell-mediated graft rejection, and prevention of recurrent glomerular disease in the allograft. The effects of rituximab on B-cell populations may last up to 9 months. During the early use of this agent for the treatment of non-Hodgkins lymphoma (NHL), no definite increased risk of infections was observed except when used for HIV-associated lymphoma, particularly with low CD4 counts
(
114,
115). Rituximab leads to a decline in neutralizing antibodies that maintain virologic control in patients with chronic HBV infection and those with isolated core HBV antibody positivity. Reactivation is known to occur as long as 1 year after the last dose of rituximab, and screening for HBV infection with serologic testing for hepatitis B surface antigen and hepatitis B core antibody and antiviral prophylaxis are routinely recommended (
116,
117,
118,
119,
120,
121). Rituximab use has also been associated with persistent and relapsing infection with
Babesia microti (
122). The treatment of babesiosis in this setting can be particularly challenging, requiring long-term antibiotics and risk of emergence of resistance. Other infections with significant association with rituximab administration include enterovirus 71 meningoencephalitis (
123), CMV (
124,
125,
126), and
Polyomavirus infections (JC and BK) (
127,
128,
129,
130,
131).
Corticosteroids
The effects of corticosteroids can be divided into two categories: an immunosuppressive effect and an anti-inflammatory one. The key immunosuppressive effect of corticosteroids is the inhibition of T-cell activation and proliferation (thus blocking clonal expansion in response to antigenic stimulation). This is accomplished through the suppression of IL-2 and other pro-inflammatory cytokines. The end result is a striking inhibition of cell-mediated immunity (CMI). The infections promoted by this impairment include herpes group viruses, hepatitis viruses, fungi, mycobacteria, and bacteria that persist intracellularly (e.g.,
Listeria spp. or
Salmonella spp.) (
132).
The anti-inflammatory effects of corticosteroids include the following: inhibition of proinflammatory cytokines; inhibition of the ability of polymorphonuclear leukocytes to accumulate at sites of infection and inflammation; inhibition of the proinflammatory arachidonic acid metabolites (e.g., prostaglandins, thromboxane, leukotrienes, and platelet-activating factor); and inhibition of mediators of vasodilatation, including the inducible form of nitric oxide synthase, thus decreasing macrophage nitric oxide production, endothelial permeability, and microvascular leak.
In addition, corticosteroid-induced dermal atrophy may predispose a patient to more catheter-related infections.
Calcineurin Inhibitors
These drugs (cyclosporine and tacrolimus) exert their effects through a complex signaling pathway that results in the inhibition of the transcriptional activation of genes required for T-cell activation, proliferation, and function. This results in the inhibition of a large number of proinflammatory cytokines, with the most important of these effects being the blockade of essential functions of IL-2. The infectious disease consequences of these drugs are direct results of these mechanisms: a dose-related inhibition of microbial-specific T-cell cytotoxic activity, thus promoting the herpes group viruses, fungal, mycobacterial, and other intracellular infections. The key toxicities of the calcineurin inhibitors are injury to the kidneys and hypertension (
22,
42,
49). Posterior reversible encephalopathy syndrome (PRES) is a serious neurologic complication associated with cyclosporine use in transplant recipients (
133,
134,
135,
136).
Sirolimus (Rapamycin)
Rapamycin’s targets include RAFT1/FRAP proteins in mammalian cells, which are associated with cell cycle phase G1. Rapamycin is less potent than the other drugs in terms of inhibition of cytokine synthesis, but has potentially useful activity in inhibiting immunoglobulin synthesis and growth factor synthesis, and potentially useful effects in protecting against chronic allograft injury. At present, the primary use of rapamycin is in combination with cyclosporine, thus permitting lower doses of cyclosporine with less renal toxicity (
45,
46,
47). The major difficulties in the use of rapamycin include pneumonitis (clinically indistinguishable from PCP), aphthous ulcers, thrombotic microangiopathy, and significant drug-drug interactions (
49,
137).