Infection in the solid organ transplant recipient may be the result of either progression to symptomatic clinical infection with organisms with which the recipient is already colonized or latently infected prior to transplantation or new acquisition of a pathogen with subsequent progression to active infection. The latter may result from exposure to contaminated healthcare environments or equipment, colonized or infected persons (including healthcare personnel [HCP], visitors, and other patients), food, water, or air or arise from the transplanted organ itself. Thus, infection prevention in the solid organ transplant recipient is dependent upon the infection prevention program and the clinical teams providing pre- and posttransplant care to the patient.
Donor and Recipient Screening
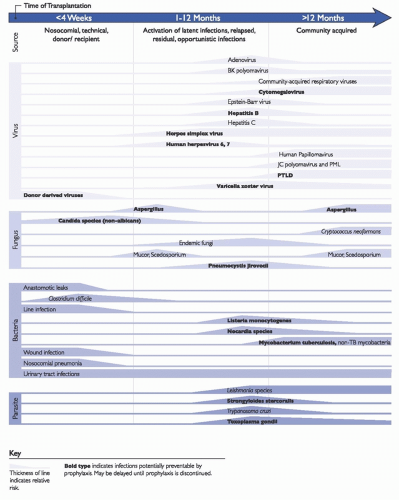
Infectious disease screening of the donor and recipient is performed to determine the acceptability of and risks associated with the organ and the eligibility of the potential organ recipient and to aid in developing an infection risk mitigation plan for the recipient. Recommendations for donor and recipient screening have been developed by several organizations.
2,3 Recommendations include screening for viral (such as human immunodeficiency virus [HIV], hepatitis B and C viruses, CMV, Epstein-Barr virus [EBV], and West Nile virus), parasitic (eg,
Toxoplasma gondii,
Trypanosoma cruzi, and
Strongyloides stercoralis), and relevant endemic fungal (eg,
Coccidioides) infections. Screening for tuberculosis and syphilis is routinely required for deceased donors but recommended only for living donors deemed to be at increased risk of infection with these organisms. Blood and urine cultures are routinely obtained from deceased donors, and respiratory cultures are additionally obtained from lung donors.
Recipient screening includes a thorough history and physical exam as well as laboratory testing. Laboratory testing includes screening for latent viral infections (ie, HIV, hepatitis B and C, CMV, EBV), relevant parasitic infections (eg, T gondi in all transplant candidates and S stercoralis and T cruzi if the candidate is from an endemic area), bacterial infections (tuberculosis, syphilis), and relevant endemic fungal infections (eg, Coccidioides in those from endemic areas). Additionally, screening for immunity to varicella-zoster virus (VZV), measles, mumps, and rubella is recommended with provision of pretransplant vaccination to nonimmune persons, if possible. Donor and recipient screening and subsequent management are typically coordinated by the organ procurement organization and the clinical team(s) providing care to the patient in the pretransplant period. However, reactivation of some latent infections, such as Mycobacterium tuberculosis and VZV, has important implications for infection transmission to other patients and HCP and will thus be addressed later in this chapter.
Transmission-Based Precautions
Implementation of transmission-based precautions (eg, contact, droplet, and airborne precautions) for transplant candidates and recipients with confirmed or suspected colonization or infection with certain pathogens can reduce the risk of spread of these pathogens within the transplant unit or ward. In general, recommendations for the use and implementation of transmission-based precautions are the same as those for other patient populations.
4 One exception to this, particularly relevant to lung transplantation, is a recommendation from the Cystic Fibrosis Foundation that calls for the implementation of contact precautions for all patients with CF, regardless of respiratory tract culture results.
6Contact precautions, which include the use of a cover gown and gloves by HCP providing care to the patient and, preferably, a single-patient room, are intended to reduce the risk of transmission of pathogens that are spread by direct contact with contaminated surfaces and other fomites or via the hands and/or clothing of HCP that have
become contaminated during the care of patients colonized or infected with the pathogen. Contact precautions are recommended in the setting of excessive wound drainage, fecal incontinence or other release of body fluids causing an increased potential for environmental contamination, infection with certain gastrointestinal pathogens (eg,
C difficile) and respiratory viruses (eg, parainfluenza viruses, respiratory syncytial virus [RSV]), and for patients colonized or infected with “multidrug-resistant organisms” (MDROs).
4,7,8 The latter group typically represents bacterial pathogens that demonstrate resistance to one or more classes of antimicrobial agents. The U.S. Centers for Disease Control and Prevention (CDC) isolation guidelines recommend the use of contact precautions for patients colonized or infected with MDROs that, based on local, state, regional, or national recommendations, are judged by the infection prevention program to be of clinical and epidemiologic significance.
4 Historically, these MDROs have included methicillin-resistant
Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and a variety of antibiotic-resistant gram-negative organisms, such as extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae and multidrug-resistant strains of
Acinetobacter baumannii,
Burkholderia cepacia, and
Pseudomonas aeruginosa. Contact precautions have also been recommended for more recently emerged MDROs, such as carbapenem-resistant and carbapenemase-producing Enterobacteriaceae (CRE and CPE, respectively) and
Candida auris.9,10 Within the infection prevention community, however, there is a lack of agreement regarding the impact of contact precautions on prevention of MDRO transmission outside of outbreak settings, particularly for VRE and MRSA. When determining the MDRO for which contact precautions will be implemented, local epidemiology, rates of adherence to basic infection control measures (eg, hand hygiene, environment and equipment cleaning,
and disinfection), prevalence of other pathogens of equivalent or greater epidemiologic significance, and facility infrastructure and resources (eg, availability of single-patient vs multiple-patient hospital rooms) should be considered. A recently published expert guidance document provides recommendations for the duration of contact precautions for various MDRO based on patient-specific factors such as time elapsed since the organism was last detected in a clinical specimen, results of screening samples, and antimicrobial therapy as well as institutional factors such the prevalence and incidence of the organism within the facility.
11As compared to contact precautions, the indications for and appropriate duration of droplet and airborne precautions are fairly straightforward and less controversial. Common indications for droplet precautions among solid organ transplant recipients include a number of respiratory viruses (eg, influenza, rhinovirus) and
Bordetella pertussis.4 It should be noted that viral shedding following infection with respiratory viruses may be prolonged in immunocompromised individuals, including solid organ transplant recipients. Airborne precautions are recommended for all patients with confirmed or suspected infection with organisms transmitted by the airborne route, such as
M. tuberculosis, VZV, and measles virus. The immunocompromised status of a transplant recipient should not prevent timely implementation of airborne precautions when such an infection is suspected.
The CDC isolation guidelines are primarily focused on interactions between patients and HCP and thus do not explicitly address the implementation of transmission-based precautions by visitors to patients who are on such precautions.
4 The use of transmission-based precautions by these persons, however, may be appropriate in some circumstances in order to protect the visitor from infection and/or to reduce the risk that the visitor will contribute to transmission of the pathogen(s) of concern. More recent guidance from the Society for Healthcare Epidemiology of America (SHEA), however, addresses this important issue.
12 Foremost, the performance of hand hygiene by visitors prior to entering the patient room and immediately upon leaving the room is recommended. Additionally, surgical masks are recommended for visitors of patients on droplet precautions and airborne precautions. The use of an N95 respirator is provided as an alternative for visitors of patients on airborne precautions. Adherence to contact precautions (ie, use of a gown and gloves) is suggested for visitors of patients with enteric pathogens, such as
C difficile and norovirus, or extensively drug-resistant gram-negative organisms, such as CPE, and during outbreak situations. Contact precautions are not recommended for visitors of patients with MRSA or VRE in most circumstances. However, the authors suggest special consideration of this practice if the visitor will be interacting with multiple patients, which may occur within the solid organ transplant population due to longer length of stay and the development of relationships among patients and their families.
Environmental Infection Control
The healthcare environment serves as an important reservoir for transmission of pathogens (see also
Chapters 42 and
43). Potential environmental sources of pathogen transmission include surfaces, furniture, medical equipment, air, water, linens, flowers, and plants.
Contaminated Surfaces and Equipment Contamination of the patient care environment and medical equipment is common
18,19,20 and has been associated with infection transmission in healthcare facilities, including outbreaks among solid organ transplant recipients.
21 This pathogen transmission can occur by direct contact with the contaminated surface or equipment or indirectly by contaminated hands of HCP. Environmental contamination can persist even after daily or discharge room cleaning.
22 In fact, several studies have observed an increased risk of acquisition of pathogens, including MRSA,
C difficile,
P aeruginosa,
A baumannii, and VRE, among patients in hospital rooms in which the previous room occupant was colonized or infected with the pathogen.
23,24,25,26 Rather than resistance to the disinfectants used during hospital room cleaning, this persistent environmental risk is more commonly thought to be the result of deficiencies in the cleaning and disinfection process.
27 Studies have demonstrated, however, that the thoroughness of environment cleaning and disinfection can be improved with multifaceted interventions that include education, performance feedback, and barrier reduction.
28,29,30 Some of these studies have further observed an association between improved thoroughness of cleaning and reduced rates of healthcare-associated infection and pathogen acquisition.
28,30 Thus, partnership with the environmental services department in order to assess and optimize the thoroughness of daily and discharge environmental cleaning and disinfection is an important component of the infection prevention program. Additionally, patient care equipment that is shared among patients must be appropriately cleaned and disinfected between uses. Dedicating equipment to individual patients, when feasible, can reduce the potential risk of pathogen transmission via medical equipment. For equipment that cannot be dedicated, collaboration with the clinical teams that use the equipment, and that are thus responsible for its cleaning and disinfection, is critical.
Air Air can serve as a source of pathogen transmission in the solid organ transplant population. In addition to its role in person-to-person transmission via respiratory droplets and aerosols (see section on Transmission-Based Precautions), hospital air can also disseminate organisms, such as fungi, that are present within the environment. There are numerous examples of outbreaks of infections due to fungal pathogens, such as
Aspergillus species, among solid organ transplant recipients in the setting of construction, renovation, and demolition within healthcare settings.
31,32 Even in the absence of construction or demolition, however, environmental fungi can be transmitted via the air within a healthcare facility. For example, a cluster of three healthcare-associated cases of mucormycosis among heart and lung transplant recipients was attributed to placement of patients in a negative-pressure room that was adjacent to a carpeted hallway and family waiting room.
33 It was hypothesized that frequent opening of the door leading to the hallway and the negative airflow of the patient room allowed dust and fungal spores to enter the patient room. Of note, none of these patients had a clinical indication for placement in a negative airflow room. Although current guidelines do not recommend routine use of a protective environment for solid organ transplant recipients, other measures are critical to minimize the risk of airborne transmission of fungal pathogens to solid organ transplant recipients. These measures include maintenance of ventilation systems to ensure that air filtration, temperature, and humidity continuously meet recommended or required specifications; performance of an infection control risk assessment (ICRA) prior to all construction, demolition, and renovation projects with implementation of appropriate dust control strategies; rapid recognition and remediation of water damage; and use of negative-pressure rooms for solid organ transplant recipients only when clinically indicated.
34
Water Hospital water systems can serve as a reservoir for waterborne pathogens with subsequent transmission to and infection of susceptible patients, such as solid organ transplant recipients. Depending on the organism, routes of transmission may include ingestion, inhalation, or direct contact with mucous membranes and nonintact skin. Numerous factors contribute to the risk of transmission, including the quality of water provided to the facility, the concentration of disinfectant(s) with which the water is treated (eg, chlorine), biofilm within the water distribution system, stagnant water (eg, in the setting of low flow, infrequent use, or dead legs within the distribution system), and water temperature. Patient-level factors, such as the degree of immunosuppression and underlying diseases that are relatively common among solid organ transplant recipients (eg, diabetes, renal failure, hepatic failure, chronic lung disease), also contribute to the risk. In addition to potable water, aerosolization of water from cooling towers can lead to pathogen transmission if the water is contaminated. Among the pathogens that can be transmitted by a healthcare facility’s potable water distribution system,
Legionella is perhaps the most commonly recognized.
Legionella infection typically results from inhalation of aerosolized water contaminated with
Legionella bacteria. Aspiration of contaminated water can also lead to
Legionella infection. In the United States, national surveillance data suggest that about 20% of cases of legionellosis occur in healthcare settings.
35 Healthcare-associated transmission has been attributed to exposure to aerosolized contaminated potable water through showering and use of faucets, respiratory therapy equipment, and humidifiers and to inhalation of aerosols generated by contaminated cooling towers. Immunocompromised patients, particularly those with compromised cell-mediated immunity such as solid organ transplant recipients, are among the populations at highest risk of developing legionellosis. Healthcare-associated outbreaks of legionellosis linked to contaminated hospital
water systems have been reported among adult and pediatric solid organ transplant recipients.
36,37,38
The CDC’s Guidelines for Environmental Infection Control in Health-Care Facilities
34 and Guidelines for Preventing Healthcare-Associated Pneumonia
39 provide recommendations for preventing Legionnaires disease in hematopoietic and solid organ transplant units. Additionally, the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) publishes a standard that provides comprehensive information and requirements for water management programs.
40 The CDC has developed a toolkit to assist in the development of a facility’s water management program.
41 Components of a
Legionella control program for the solid organ transplant unit include clinical surveillance to identify cases of legionellosis, minimizing conditions that promote growth of
Legionella species within the water distribution system (eg, maintenance of circulating water temperatures outside of the range that is optimal for
Legionella growth and replication (ie, maintain temperature <77°F [25°C] or >108°F [42.2°C]), prevention of water stagnation), periodic water sampling to assess for the presence of
Legionella species, and decontamination of the water system when established tolerance thresholds for
Legionella are exceeded. Other recommended preventive measures include monthly disinfection of shower heads and tap aerators, if used, using a chlorine-based EPA-registered product. If clinical cases of possible healthcare facility-associated legionellosis are identified, an epidemiologic and environmental investigation should be initiated. If
Legionella contamination of the hospital’s water system is found or suspected, additional interventions are necessary. While the water supply is being disinfected and/or while awaiting results of testing to determine if the water is potential ongoing source of
Legionella, risks to patients can be minimized by restricting the use of water from faucets in patient care rooms, restricting transplant recipients and other severely immunocompromised persons from taking showers, using noncontaminated water for sponge baths, and providing an alternative source of water (eg, sterile water) for tooth brushing, drinking, and flushing nasogastric tubing.
Healthcare facility water distribution systems may also serve as a source of transmission of other pathogens. In a review of 134 CDC consultations related to healthcareassociated infections caused by water-related organisms other than
Legionella between 2014 and 2017, nontuberculous mycobacteria (NTM) accounted for the greatest proportion of consultations (29.9%).
42 NTM were followed in frequency by several gram-negative organisms, including
P aeruginosa,
Burkholderia species, and
Enterobacter species. In a systematic review of 21 articles reporting nosocomial waterborne NTM infections, sources of transmission included tap water from showers and sinks leading to infection via central venous catheters or surgical wounds and use of nonsterile water during procedures when sterile water should have been used (eg, rinsing reusable medical equipment).
43 Like nosocomial
Legionella infections, these NTM infections were associated with deficiencies in the facility’s water distribution system or its maintenance, such as water stagnation, low concentrations of chlorine, water tank sediment, and interruptions in the water supply. More recently, an international outbreak of
M chimaera infections attributed to the use of contaminated heater-cooler devices used in cardiothoracic surgery, including heart/lung transplant procedures, has been described.
44,45 This outbreak was caused by contamination of the devices at the manufacturing plant due to the use of contaminated tap water with subsequent generation of NTM-contaminated aerosols during intraoperative use of the machine. The contaminated aerosol presumably resulted in contamination of the sterile field, graft materials, and/or open chest cavity. The manufacturer, CDC, and the U.S. Food and Drug Administration (FDA) have issued guidance for facilities that use the implicated heater-cooler devices, including specific recommendations for patient notification and management of the implicated devices.
46 (Note: At the time of writing, this is an ongoing and evolving situation.) This outbreak highlights the importance of avoiding contamination of these and other potentially aerosol-generating devices within the hospital.
Plants and Flowers Plants, soil, and water may serve as reservoirs for fungi (eg,
Aspergillus and
Fusarium species) and bacteria (eg,
P aeruginosa) that can act as pathogens in immunocompromised hosts, such as solid organ transplant recipients. Thus, fresh and dried flowers and potted plants should not be allowed in patient care areas where immunosuppressed patients are located.
34
Healthcare Linens Improper processing, transport, and storage can result in microbial contamination of healthcare linens. Outbreaks of fungal (eg,
Rhizopus species) and bacterial (eg,
Bacillus species) infections among immunocompromised patients, including solid organ transplant recipients, associated with contaminated linens have been reported.
47,48,49,50 Contamination may result from one or more deficiencies in laundering (including inadequacies in air handling, dust control, and cleaning within the laundry facility; suboptimal water temperature; improper chemical composition during the laundering process; and incomplete drying of linens), transportation (eg, inadequate cleaning and disinfection of linen carts), or storage. Regardless of whether the hospital’s linens are processed on-site or at an off-site facility, adherence to recommended healthcare linen reprocessing, transportation, and storage practices is necessary.