Dr. John Snow (1813-1858) is best known for his groundbreaking investigation to identify and mitigate the source of cholera outbreaks, the public Broad Street water pump, in London in 1853. Less known is that Snow’s primary specialty was anesthesia. His studies of the safe use of volatile agents helped the practice gain acceptance, and he administered chloroform analgesia to Queen Victoria for the birth of her eighth and ninth children.
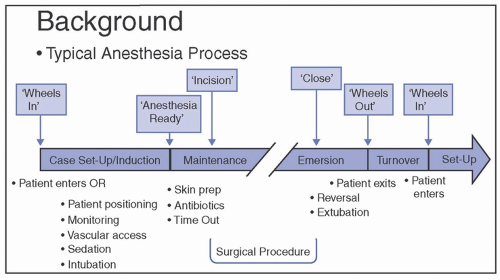
1Infection prevention is often difficult to practice in the modern anesthesia work area. Both fields have evolved over time and produced innovations and advances to prevent patient harm. However, not all developments in anesthesia have addressed infection prevention, and similarly, many infection prevention processes do not take anesthesia into account. Significant advances in infection prevention, such as in prevention bundles, are meant to be implemented in a clinic or inpatient room setting. The World Health Organization’s (WHO) 5 moments of hand hygiene
2 are difficult if not impossible to follow during anesthesia induction. As Dr. Snow did not combine his two areas of interest, so too have the respective fields not aligned practices effectively.
While numerous guidelines have been published on infection prevention in anesthesia practice,
3,4,5,6,7 a lack of uniform knowledge and implementation of recommendations persists. In a recent survey of members of the American Academy of Anesthesiologist Assistants (AAAA), subsets of the American Association of Nurse Anesthetists (AANA), and the American Society of Anesthesiologists (ASA), only 87% of respondents reported always wearing gloves for airway management.
8 In 2014, safe use of medication vials was the subject of a Joint Commission (TJC) Sentinel Event Alert
9 after a Centers for Disease Control and Prevention (CDC) assessment of ambulatory surgery centers showed widespread deficiencies, including routine use of single-dose medication vials for multiple patients in 28% of facilities surveyed. Additionally, surveys of U.S. healthcare providers,
10,11 New York State anesthesiologists,
12 New Zealand anesthesiologists,
13 and certified nurse anesthetist students
14 reported improper practices despite knowledge of associated risks and existence of guidance. Despite widespread publicity, practice gaps likely persist. In the more recent survey referenced above, 92% reported always disposing of a needle or syringe between every patient, but only 43% reported never using multidose vials for more than 1 patient.
8This apparent disconnect has led some authors to conclude that attention to and redesign of the anesthesia work area, through collaboration between anesthesia and prevention professionals, is necessary to align the two practices. Notably, anesthesia professionals play a crucial role in surgical site infection (SSI) prevention, such as ensuring proper dosing and timing of antimicrobial prophylaxis and maintaining normoglycemia and normothermia,
15 many of which carry a risk of disease transmission. This chapter reviews the evidence for pathogen transmission in the
operating room (OR) and anesthesia work area that can occur during delivery of preventive and other cares. The available evidence and current recommendations for preventing transmission are summarized.