Author (year, country) |
Patient Population |
No. |
Primary Site(s) |
Species |
Probable Source |
Control Measures Recommended or Applied |
Pelaez et al. (2012, Spain) (88) |
Cardiac ICU |
7 |
LRTI, mediastinitis |
A. fumigatus |
Construction |
ICU closed for 1 month with HEPA filters installed |
Mueller et al. (2009) (370) |
Transplant recipients |
5 |
Transplanted organs |
A. fumigatus |
Donor |
Rapid notification of recipients |
Kidd et al. (2009, Australia) (371) |
Hematology |
3 |
LRTI |
A. fumigatus |
Undetermined |
Improved infection control measures; ICU closed and cleaned; faulty structures replaced |
Chang et al. (2008, Australia) (89) |
Hematology |
6 LRTI |
A. fumigatus |
Construction |
Unit relocation; impermeable barriers at construction site; N95 face masks for patients during transport; voriconazole prophylaxis for other high-risk patients; relocation of patient garage |
Rodigo et al. (2007, Sri Lanka) (115) |
Obstetric |
6 |
Meningitis |
A. fumigatus |
Contaminated syringes and spinal needles, poorly maintained storage area |
Withdrawal and incineration of all unused syringes |
Raviv et al. (2007, Israel) (90) |
Transplant |
8 |
LRTI, endophthalmitis |
A. fumigatus, A. flavus, A. terreus, A. niger |
Construction |
Not reported |
Saracli et al. (2007, Turkey) (92) |
Ophthalmology |
3 |
Endophthalmitis |
A. ustus |
Construction |
Not reported |
Kronman et al. (2007, USA) (372) |
Pediatric cardiac ICU |
3 |
SSI |
A. fumigatus |
Undetermined |
Cleaning of all involved rooms; HEPA vacuuming areas; mold inspection; evaluation and improvement of infection control practices |
Panackal et al. (2006, USA) (91) |
Hematology |
6 |
SSI, LRTI |
A. ustus |
Construction |
Not reported |
Heinemann et al. (2004, Belgium) (116) |
Cardiac ICU |
9 |
SSI |
A. flavus |
Water damage |
Cleaning; disinfection of ORs; repeat environmental surveys |
Panackal et al. (2003, USA) (81) |
Renal transplant |
7 |
LRTI |
A. fumigatus |
Construction |
Impermeable barriers; HEPA filters in HVAC system; N95 respirator use during patient transport; reduce traffic; designated elevator for construction workers |
Myoken et al. (2003, Japan) (373) |
Hematology |
6 |
Stomatitis |
A. flavus |
Undetermined |
Not reported |
Lutz et al. (2003, USA) (94) |
Surgical |
6 |
SSI |
A. fumigatus, A. flavus |
Air-handling system, moist insulation |
Remediation of air-handling unit: remove interior insulation; coat units with fungicide; cleaning diffusers |
Pegues et al. (2002, USA) (118) |
Transplant ICU |
3 SSI, LRTI |
A. fumigatus |
Debriding and dressing wounds |
Minimize disruption of wound; keep wound covered |
Hahn et al. (2002, USA) (374) |
Hematology-oncology |
10 |
LRTI |
A. flavus, A. niger |
Contaminated wall insulation from non-BMT wing |
Impermeable barriers; decontamination of wall insulation; HEPA filters in non-BMT wing |
Oren et al. (2001, Israel) (80) |
Hematology-oncology |
10 |
LRTI |
Not reported |
Construction, renovation |
Prophylaxis with low-dose systemic and inhaled amphotericin B and systemic; locate patients in special ward with HEPA-filtered air |
Lai (2001, USA) (76) |
Hematology-oncology |
3 |
LRTI |
A. flavus |
Construction |
BMT unit closed for 2 weeks; air intake ducts cleaned; filters and prefilters replaced; impermeable barriers; alarm installed and air pressure made negative in stairwell leading to construction site; edge guards around doors to anterooms; carpeting replaced by vinyl flooring; special unit that allowed breathing filtered air during patient transport |
Burwen (2001, USA) (70) |
Hematology-oncology |
6 |
LRTI |
A. flavus |
Construction |
Identify high-risk patients and locate in rooms with HEPA or laminar airflow |
Thio et al. (2000, USA) (141) |
Hematology-oncology |
21 |
LRTI |
A. flavus |
Adjacent connected hospital with higher air pressure than unit |
Stop elective admissions; plants and produce prohibited in patient rooms; doors connecting to adjacent hospital engineered to close automatically; wipe or mop all surfaces wet; maximize pressure relationships; doors to individual patient rooms kept closed; N95 masks for neutropenic patients during transport; resealed windows; employee entrance near construction area closed |
Gaspar et al. (1999, Spain) (71) |
Hematology-oncology |
11 |
LRTI |
Not reported |
Construction |
Sealing of construction area; patients relocated |
Tabbarra and al Jabarti (1998, Saudi Arabia) (85) |
Cataract surgery |
5 |
Eye infection |
A. fumigatus |
Construction |
Not reported |
Singer et al. (1998, Germany) (375) |
NICU |
4 |
Skin infection |
A. fumigatus, A. flavus |
Latex finger stall attached to penis to collect urine samples from male preterms |
Removal of finger stalls |
Loo et al. (1996, Canada) (78) |
Hematology-oncology |
36 |
LRTI, sinusitis |
A. flavus, A. fumigatus |
Construction |
Portable HEPA filter units; copper-8-quinolinolate-formulated paint to walls, doors, baseboards, vents, and above false ceiling; windows sealed; replacement of perforated ceiling tiles with nonperforated, vinyl-faced aluminum tiles; horizontal dust-accumulating blinds replaced with roller shades; temporary relocation of patients |
Leenders et al. (1996, Netherlands) (376) |
Hematology-oncology |
5 |
LRTI, sinusitis, eye infection, mastoiditis |
A. fumigatus, A. flavus |
No single source |
Reinforce policies for maintaining HEPA-filtered rooms; keep windows closed at all times |
Bryce et al. (1996, Canada) (68) |
Surgical and burn units |
4 |
Skin infection |
Not reported |
Construction, contaminated packages of dressing supplies |
Sealing off construction area; supply room damp-dusted and vacuumed; boxes and supplies wiped with cloth with buffered bleach |
Tang et al. (1994, UK) (377) |
Renal transplant unit |
2 |
LRTI |
A. fumigatus |
Construction |
Impermeable barriers |
Iwen et al. (1994, USA) (74) |
Hematology-oncology |
5 |
LRTI |
A. fumigatus, A. flavus |
Construction |
Multiple measures prior to construction; environmental monitoring with gravity air-settling plates during construction; guiding additional measures |
Buffington et al. (1994, USA) (69) |
Hematology-oncology |
7 |
LRTI |
A. fumigatus, A. flavus |
Construction |
HEPA filters; proper pressure relationships; physical barriers; area decontamination |
Tritz et al (1993, USA) (378) |
Hematology-oncology |
4 |
LRTI |
A. terreus, A. fumigatus |
Not reported |
Not reported |
Flynn et al. (1993, USA) (93) |
Hematology-oncology, medical ICU |
4 |
LRTI |
A. terreus |
Construction, improper air pressure relationships |
Reestablish positive pressure and unidirectional airflow in ICU |
Richet et al. (1992, USA) (379) |
Open heart surgery |
6 |
SSI |
A. fumigatus |
Undetermined |
Not reported |
Pla et al. (1992, Spain) (380) |
Liver transplant |
2 |
SSI |
A. fumigatus |
Contaminated operating room |
Not reported |
Loosveld et al. (1992, Netherlands) (381) |
Hematology-oncology |
6 |
LRTI |
A. fumigatus |
Cracked plasterwork |
Renovation of plastering; HEPA filters installed in each room; intensified cleaning procedures |
Humphreys et al. (1991, UK) (99) |
General ICU |
6 |
LRTI |
A. fumigatus, A. flavus |
Perforated metal ceiling with contaminated insulation |
Extensive cleaning of ICU; temporary relocation of patients; old ICU replaced by new ICU with enhanced ventilation system and without false ceilings |
Arnow et al. (1991, USA) (96) |
Hematology-oncology, solid organ transplant |
15 |
LRTI |
A. flavus, A. fumigatus |
Contaminated air filters |
Remediate air-handling unit; removal of contaminated air filters; damp-wipe surfaces in patient areas; remove carpet |
Weber et al. (1990, USA) (86) |
Hematology-oncology |
18 |
LRTI |
Not reported |
Construction |
Not reported |
Mehta (1990, India) (382) |
Open heart surgery |
4 |
Endocarditis |
A. fumigatus |
Air-handling system, broad-spectrum antibiotics |
Weekly scrub of V filters and cooling coils; replacement of filters with series of prefilters and HEPA filters; increase air changes; restriction of broad-spectrum antibiotics |
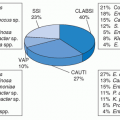
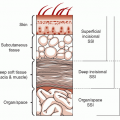
LRTI, lower respiratory tract infection; SSI, skin and soft-tissue infection. |