Neutrophils are well known to function as the first line of defense against invading pathogens, principally bacteria and fungi, but also viruses.
1 These cells, together with monocytes, macrophages, and dendritic cells (DCs), feature the characteristic properties of the “professional” phagocytes and utilize several effector mechanisms to destroy pathogens, including the generation of massive amounts of reactive oxygen species (ROS) in combination with the discharge of many potent antimicrobial enzymes or factors.
1 Because of their powerful microbicidal equipment, neutrophils are often depicted as harmful cells that can cause damage to the surrounding tissues during acute inflammation (as observed in those inflammatory diseases dominated by neutrophils).
1 Nonetheless, extensive research performed in the last 20 years has recognized neutrophils as highly versatile and sophisticated cells displaying a significant synthetic capacity as well as an important role in linking the innate and adaptive arms of the immune response.
2,3
Neutrophil Generalities
Neutrophils are the most abundant (40% to 70%) circulating leukocyte type in human blood, normally present at 2.5 to 7.5 × 10
9 cells/L.
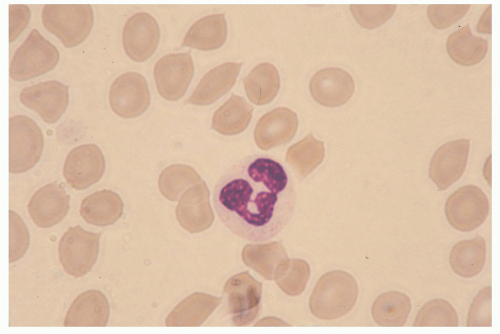
4 Morphologically, these cells can be identified by the peculiar shape of their nucleus, which is polymorphous and usually consists of three to five sausage-shaped masses of chromatin connected by fine threads
(Fig. 20.1). Mature neutrophils are terminally differentiated, nondividing cells that develop and mature in the bone marrow from pluripotent CD (cluster of differentiation)34-positive (CD34+) stem cells, under the regulatory effects of several colony-stimulating factors (CSFs), including granulocyte-macrophage CSF (GM-CSF), granulocyte CSF (G-CSF), interleukin (IL)-3, and IL-6.
4 Neutrophils can be cytofluorimetrically identified by their characteristic morphology (high side scatter) and expression pattern of plasma membrane proteins such as CD66b, CD11b, CD15, and CD16, in conjunction with the lack of expression of CD2 and CD19. In humans, circulating polymorphonuclear leukocytes are 10 to 20 &mgr;m in diameter, display a half-life previously thought to correspond to 7 to 12 hours, but more recently reevaluated and extended to up to 90 hours,
5 and exist in a dynamic equilibrium with a “marginated” pool that is sequestered within the microvasculature of many organs.
1,4 In the resting uninfected host, the peripheral neutrophil population is maintained within a constant number by several mechanisms. One of them consists in programming neutrophils to spontaneously undergo apoptosis
1,3 to be, in turn, cleared by tissue macrophages located in the bone marrow, spleen, and liver.
6 In this latter context, a feedback loop involving an IL-23/IL-17/G-CSF axis, crucial for the regulation of neutrophil production, has been recently identified in mice.
7 According to this model, the uptake of apoptotic neutrophils by macrophages and DCs would determine a downregulation of their IL-23 production. Consequently, the Th17 subset of proinflammatory T-lymphocytes would be poorly sustained and thus much less IL-17A would be generated. As IL-17A positively regulates fibroblast- and endothelial cell-derived G-CSF, which is essential for controlling both granulopoiesis and neutrophil survival, the final outcome of this circuit—triggered by the massive neutrophil
apoptosis at peripheral sites—would consist in a decrease in the levels of neutrophils released from the bone marrow.
7 On the other hand, the number of circulating neutrophils can dramatically increase (even up to 10-fold) under acute inflammatory conditions (eg, during a bacterial infection), from accelerated neutrophil production and release from the bone marrow.
4 Moreover, even the lifespan of neutrophils is significantly extended under inflammatory conditions, as various host- and pathogen-derived mediators such as G-CSF, GM-CSF, interferon (IFN)-&ggr;, tumor necrosis factor (TNF)-&agr;, lipopolysaccharide, and nucleic acids inhibit neutrophil apoptosis and hence prolong their survival.
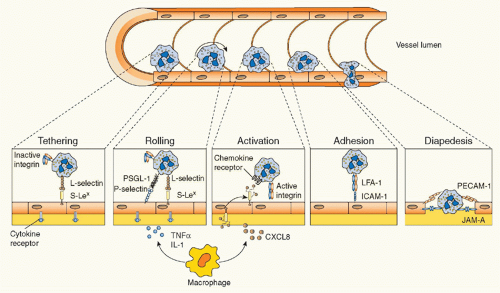
8During an acute inflammatory response, neutrophils are rapidly recruited to the site of injury by a coordinated sequence of events that begin with the elaboration of various mediators able to specifically promote their migration from the intravascular compartment. Mediators derive from numerous sources (tissue macrophages, endothelial cells, activated plasma components) and include vasoactive amines, proinflammatory lipids, small polypeptides, chemotactic factors, and cytokines such as TNF&agr;, IL-1&bgr;, or IL-17A (the latter being one of the most abundant products of Th17 cells).
1,3 Chemotactic factors, in particular, are generated in temporally distinct waves and include C5a, leukotriene-B
4 (LTB
4), formyl-Met-Leu-Phe (fMLF), as well as neutrophil specific chemokines, such as CXCL8/IL-8, CXCL1/GRO&agr;, CXCL5/epithelial cell neutrophil-activating protein-78, etc.
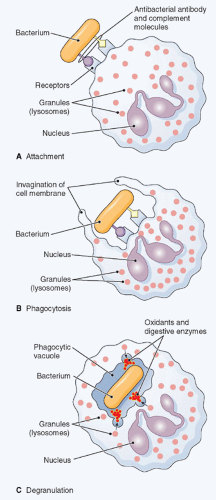
1,3 Once recruited at an inflammatory site, neutrophils function as mobile arsenals that recognize, phagocytose, and ultimately destroy their targets. If the acute inflammatory response correctly subsides, then neutrophils may actively participate in its resolution (see following discussion). If not, an uncontrolled and continuous release of the proinflammatory cargo (ROS and proteases) by neutrophils recruited at the site of infection/injury may eventually lead to destruction of bystander tissue, and thus to exacerbation of the ongoing inflammation, ultimately provoking the onset of chronic inflammatory/autoimmune diseases.
9
Neutrophil Microbicidal Mechanisms
To destroy and eliminate invading pathogens, neutrophils essentially utilize two fundamental mechanisms
10: an oxygen-dependent process that is mediated by the generation of ROS, which include O
2– (superoxide anion), hydrogen peroxide, singlet oxygen, and other products derived from the metabolism of hydrogen peroxide; and an oxygen-independent process consisting in the release into the phagocytic vacuole of lytic enzymes; and antimicrobial polypeptides stored in their intracellular granules.
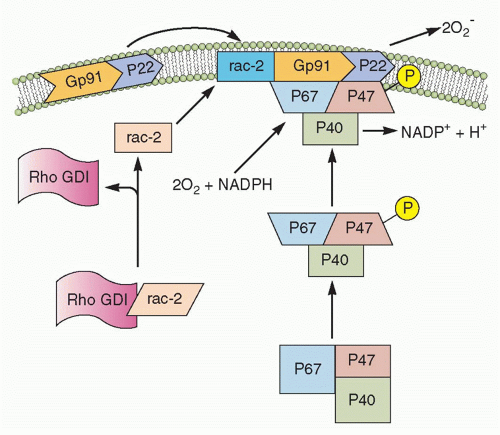
10 The oxygen-dependent process, also referred to as the “respiratory burst,” is defined as an increase of a mitochondrial independent oxidative metabolism that leads to the generation of O
2–, which occurs through the activation of the phagocytic NADPH oxidase, an enzymatic system that is unique to phagocytes (neutrophils, monocytes, macrophages, DCs, and also Eos). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a multiprotein complex formed by a flavocytochrome-b
558 (a heterodimer of gp91
phox and p22
phox chains, where
phox stands for phagocyte oxidase), three cytoplasmic components (namely p40
phox, p47
phox, p67
phox) and either Rac1 or Rac2 from the Rho family of low-molecular-weight GTPases
(Fig. 20.2). Upon cell stimulation, the cytosolic components of the complex become phosphorylated and assemble together with the cytochrome and Rac1/2 on the plasma membrane, thus forming the active enzyme that produces superoxide anion radicals, by catalyzing the transfer of electrons from NADPH to molecular oxygen (see
Fig. 20.2). O
2– is converted by superoxide dismutase into hydrogen peroxide, which, in the presence of myeloperoxidase and halogens, is then metabolized into hypochlorous acid. The latter represents one of the neutrophil’s
major weapons against microbes, as it also synergizes with granule proteins to kill pathogens in the neutrophil phagolysosome. O
2– can also reacts with other cellular radicals, such as nitric oxide, to form different species of cytotoxic oxidant, such as peroxynitrite. The critical role of NADPH oxidase and its products in host defense is best illustrated by the plight of patients with chronic granulomatous disease, in which mutations in any of the NADPH oxidase complex subunits (gp91
phox, p22
phox, p40
phox, p47
phox, and p67
phox) leads to a severe immunodeficiency characterized by defective killing of phagocytosed pathogens for the lack of ROS generation.
10 These infections typically involve microorganisms for which oxidant-mediated killing is particularly critical for effective host defense, such as
Staphylococcus aureus,
Aspergillus spp.,
Nocardia, and a variety of gramnegative enteric bacilli.
The release of potent proteolytic enzymes contained in their granules represents the other crucial mechanisms utilized by neutrophils to eliminate pathogens following phagocytosis.
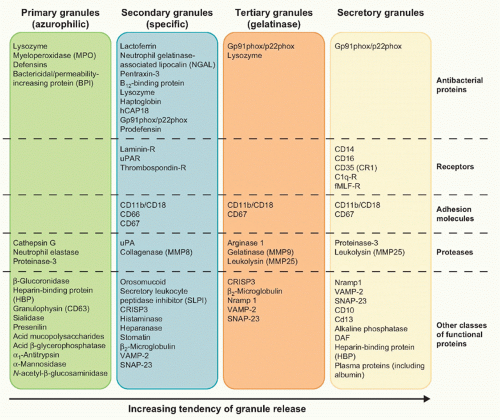
10 Neutrophil granules are subdivided into peroxidase-positive granules (based on the presence of myeloperoxidase, their marker), also called primary or azurophil granules (owing to their affinity for the basic dye azure A), and peroxidase-negative granules that include the specific (secondary) granules, the gelatinase (tertiary) granules, and the secretory vesicles
11 (Fig. 20.3). The different types of granules appear at progressive stage of neutrophil development,
11 with the primary granules, as suggested by the name, being the first ones to appear during hematopoiesis at the promyelocyte stage. As highlighted in
Figure 20.3, granules are released in a hierarchical order and under separate control by mature neutrophils, depending on the type of stimulus.
11Azurophil or primary granules undergo limited exocytosis in response to stimulation and are packaged with acidic hydrolases and antimicrobial proteins that contribute primarily to the killing and degradation of engulfed microorganisms into the phagolysosome.
11,12 These granules contain myeloperoxidase, an enzyme that catalyzes the formation of hypochlorous acid, hydrolases, lysozyme, matrix metalloproteinases, and three structurally related serprocidins (serine proteases with microbicidal activity): proteinase-3, cathepsin G, and elastase. The latter proteins can degrade a variety of extracellular matrix components, including elastin, fibronectin, laminin, type IV collagen, and vitronectin. Azurophil granules also contain antimicrobial molecules such as bactericidal/permeability-increasing protein (which is important for killing gram-negative bacteria) and &agr;-defensins, a family of small cysteine-rich antibiotic peptides with broad antimicrobial activity against bacteria, fungi, and certain enveloped viruses.
11,12Specific (or secondary) granules, which are formed at the myelocytic stage, are smaller and less dense than the azurophil ones, and contain unique constituents, such as collagenase, haptoglobin, vitamin B
12-binding protein, as well an extensive array of membrane-associated proteins including cytochromes, signaling molecules, and receptors.
11,12 Secondary granules also contain an arsenal of antimicrobial substances, such as lactoferrin, neutrophil gelatinase-associated lipocalin, lysozyme, hCAP18 (cathelicidin human cationic antimicrobial protein of 18 kDa, the proform of LL-37), and pentraxin-3. The inhibitory effect of antimicrobial proteins include depriving ions essential for microbial survival, degrading structural components of microorganisms (eg, peptidoglycan), and disrupting the integrity of target cell membrane by punching pores in the membrane or by perturbing membrane integrity. Neutrophil-specific granules also contain an important family of soluble proteinases, known as matrix metalloproteinases (MMPs), which include neutrophil collagenase-2 (MMP-8), gelatinase-B (MMP-9), and leukolysin (MMP-25). These proteinases are generally stored as inactive proenzymes and undergo proteolytic activation following granule fusion and interaction with azurophilic granule contents. Neutrophil MMPs disrupt major structural components of bacteria and/or extracellular membranes, and are therefore crucial not only for bacterial killing, but also for neutrophil extravasation and migration.
11,12Tertiary granules are produced at the metamyelocyte stage of differentiation and are smaller, lighter, and more easily exocytosed than the other granule classes.
11,12 These granules are indeed important primarily as a reservoir of matrixdegrading enzymes and membrane receptors needed during polymorphonuclear leukocyte extravasation and diapedesis. The primary constituent of tertiary granules is gelatinase, a latent metalloenzyme with the capacity for tissue destruction. Finally, the secretory vesicles are smaller than the other granules, are generated by endocytosis during the late stage
of nuclear neutrophil segmentation in the bone marrow, and are the most readily mobilizable.
11,12 These vesicles are preferentially directed to the plasma membrane, as reflected in the density of vesicle-associated membrane protein (VAMP), a fusogenic protein associated with the granule membrane. Secretory vesicles do not contain toxic substances, but mainly plasma proteins like albumin and receptors (including &bgr;
2-integrins, the complement receptor [CR]1, receptors for formylated bacterial peptides [fMLF-R], CD14, the Fc portion of &ggr;-immunoglobulins (Igs) [Fc&ggr;RIII/CD16], and the metalloprotease leukolysin). Heparin-binding protein (also known as CAP37 or azurocidin), whose release is essential for the polymorphonuclear leukocyte-induced increase in vascular permeability at the initial stage of extravasation, is also stored in the secretory vesicles.
An additional nonphagocytic microbicidial mechanism used by neutrophils to capture and destroy microbes in the extracellular space consists in the ability of neutrophils to form so-called neutrophil extracellular traps (NETs).
13 The latter structures consist of nuclear chromatin decorated with antimicrobial peptides and enzymes (eg, bactericidal/permeability-increasing protein, elastase, pentraxin3, cathepsin G, and many others) that lacks membranes and cytosolic markers.
13 NETtosis, a novel type of neutrophil death mechanism that occurs under settings of extreme neutrophil stimulation (different from necrosis, apoptosis, and also independent from caspase activation), underlies the generation of NETs.
14 Accordingly, the nuclear envelope, granules, and cell membranes gradually dissolve during NETtosis, allowing the nuclear contents to mix and condense in the cytoplasm before being released into the extracellular space.
14 NETs, in turn, bind to various grampositive and gram-negative bacteria (such as
Staphylococcus aureus,
Salmonella typhimurium,
Streptococcus pneumoniae,
and group A streptococci), as well as to pathogenic fungi (such as
Candida albicans). Similarly to what happens in the phagolysosome, the high local concentration of antimicrobial peptides and enzymes is responsible for the killing of the pathogens trapped by NETs.
13 The observation that neutrophils from patients with chronic granulomatous disease do not form NETs has suggested, on the one hand, that ROS-mediated signaling/cascades are involved in NET generation, and, on the other hand, that the lack of NETs might contribute to the pathogenesis of chronic granulomatous disease.
3,14 Whatever the case, it is noteworthy to remark that both the oxygen-dependent and -independent effector mechanisms in host defense toward pathogens are also utilized by neutrophils for their cytotoxic and tumoricidal activities.
Neutrophil Receptors
Under inflammatory conditions, neutrophils sense a wide range of extracellular ligands that, through the interaction with specific receptors, subsequently trigger a number of effector functions, including adhesion, migration, phagocytosis, survival, cell activation, gene expression modulation, target cell killing, and mediator production and release.
1,3,15 For instance, agonist-stimulated neutrophils may trigger not only degranulation, but also the release of arachidonic acid and/or other eicosanoids (eg, prostaglandin [PG]E
2), via the activation and/or the upregulation of PLA
2 and COX-2, respectively. Upon appropriate stimulation, neutrophils can also generate LTA
4 through the action of 5-lipoxygenase, as well as LTB
4 by converting LTA
4 via the action of LTA
4 hydrolase.
15 A nonexhaustive list of neutrophil receptors includes 1) receptors for proinflammatory mediators (eg, the anaphylotoxin complement component C5a, LTB
4, platelet-activating factor [PAF], substance P, and fMLF); 2) receptors for cytokines, such as IFN&ggr;, IL-1, IL-4, IL-6, IL-10, IL-13, IL-15, IL-18, TNF&agr;, G-CSF, GM-CSF, and many others; 3) receptors for chemokines, including CXCR1 and CXCR2; 4) receptors/adhesion molecules for the endothelium; 5) receptors for tissue matrix proteins; and 6) opsonin receptors, such as Fc&ggr;Rs and those for the major cleavage fragments of the complement system (see following discussion). Neutrophils also express a variety of pattern recognition receptors (PRRs), including all toll-like receptors (TLRs), with the exception of TLR3, cytoplasmic ribonucleic acid helicases involved in viral ribonucleic acid recognition such as MDA5 and RIGI, and deoxyribonucleic acid binding cytoplasmic proteins (IFI16 and LRRFIP1).
3