| Urethra |
| Pharynx |
| Cervix |
| Conjunctiva |
| Rectum |
| Vagina (in prepubescent girls) |
Acute urethritis, manifesting as some combination of urethral discharge and dysuria, is the most common presentation of disease in men, although some infected men are asymptomatic. Gram stain of urethral discharge may be used for presumptive diagnosis of gonococcal urethritis. Polymorphonuclear neutrophils (PMNs) with gram-negative, intracellular diplococci (GNID; Figure 139.1) are observed in 95% of infected, symptomatic men, and the finding is 98% specific. Observing PMN without GNID supports a diagnosis of nongonococcal urethritis (see Chapter 59, Urethritis and dysuria), but the sensitivity of GNID in asymptomatic men is only about 75%, and Gram stain cannot be used to rule out gonorrhea in these patients.
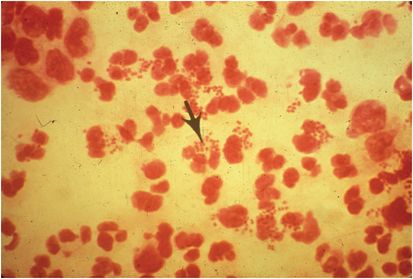
Figure 139.1 Gram stain of urethral discharge, showing gram-negative intracellular diplococci diagnostic of gonorrhea. Coincident nongonococcal urethritis cannot be ruled out. (Courtesy of Centers for Disease Control and Prevention.)
In women, the primary site of infection is the endocervix, although the organism can be recovered from the urethra and periurethral (Skene’s) and Bartholin’s glands in adults and from the vagina itself in prepubescent girls. Asymptomatic infection is more common in women than men. Gram stains of cervical smears from infected women are 97% specific for the disease when GNID are observed but only 25% to 70% sensitive.
Anorectal gonorrhea occurs in up to 40% of women with endocervical disease. It can also be an isolated finding in homosexual men who practice receptive anal intercourse. Most patients are asymptomatic, but acute proctitis may occur. Most patients with pharyngeal infection, acquired from fellatio, are also asymptomatic, but pharyngitis may occur. Gonococcal conjunctivitis in adults, usually acquired by autoinoculation from a genital focus, produces varying degrees of inflammation.
Disseminated gonococcal infection is increasingly uncommon, occurring historically in perhaps 0.5% to 3% of infected patients. It usually presents as the arthritis–dermatitis syndrome, manifesting as asymmetric, migratory polyarthritis, arthralgias, or tenosynovitis. In 75% of cases, disseminated disease is accompanied by small papules or by vesicles or pustules with an erythematous base. Hereditary deficiency of the terminal components of complement or infection with organisms resistant to the bactericidal activity of serum predisposes to dissemination. Endocarditis and meningitis are very rare complications.
Diagnosis
Definitive diagnosis requires demonstrating the presence of the organism. This is traditionally accomplished by culture of infected material, but culture has been largely supplanted by molecular techniques. Nucleic acid amplification tests (NAATs) are those most widely used clinically in the United States. The ligase chain reaction is highly specific and sensitive when used on a urethral, cervical, or vaginal specimen. However, studies on urine suggest an unacceptably low sensitivity of about 60% in women. Disadvantages of such molecular technology include the ability of the tests to detect dead organisms for perhaps as long as 2 weeks after successful treatment, lack of licensure for use on anal or pharyngeal specimens, and inability to provide a specimen for testing of antimicrobial sensitivity. As new data develop, the clinician should review the material provided with these tests for indication of their appropriate use. Although culture requires specialized collection apparatus, it is important to obtain appropriate cultures and susceptibility testing on all patients who do not improve on therapy, once reinfection and nonadherence to medication have been ruled out.
Therapy
There are several important and unique general principles to consider regarding treatment of gonococcal infections. Ideal treatment of uncomplicated infection should be: single dose, affordable, possessing a low side effect profile, and, when possible, oral. Unfortunately, due to increases in antimicrobial resistance, there is no longer a recommended first-line oral option for therapy. The treatment for uncomplicated infection is almost always empirical and performed without knowledge of antimicrobial susceptibility. There are suprisingly few bacterial infections for which in vitro resistance correlates so tightly with clinical treatment failure. Resistance has emerged to all antimicrobial classes used for treatment of gonorrhea. Historically, one can follow the rapid appearance and dissemination of organisms resistant to penicillins, sulfonamides, tetracyclines, fluoroquinolones (FQ), and, most recently, cephalosporins. The genetic chronicle of gonococcal resistance has been mediated by a series of chromosomal mutations and by acquisition of readily dissmeninated plasmids that encode for resistance genes and have the ability to transfer between strains and even genera.
The rapid rise of penicillin and tetracycline resistance was first identified in the 1970s. Resistance to both classes has been driven by chromosomal mutations and dissemination of plasmids carrying genes which encode for a β-lactamase and a protein which modifies the ribosomal target of tetracycline. Although the spread of plasmid-mediated resistance has waned over the last decade, chromosomal resistance remains an issue and, ultimately, neither drug can be reliably used for routine treament.
In the mid 1980s a single, oral dose of FQ successfully treated gonococcal urethritis in men. However, as early as 1990, resistance to ciprofloxacin was detected in N. gonorrhoeae. The prevalence of FQ resistance and the incidence of treatment failures continued to rise throughout the 1990s, initially in Southeast Asia and then in Hawaii and California. N. gonorrhoeae has many mechanisms which may contribute to FQ resistance. The primary mechanism of FQ resistance is mutation in one or both genes (parC or gyrA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





