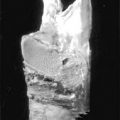
Fig. 1
Pt with visible but asymptomatic cervical multinodular goiter. Grade 3 by World Health Organization goiter classification system
World Health Organization (WHO) Grading of Thyroid Goiter
Grade 0: No goiter found, thyroid impalpable/invisible
Grade 1a: Thyroid palpable but invisible even with full neck extension
Grade 1b: Thyroid palpable in neutral position/visible with neck extension
Grade 2: Goiter visible, no palpation required to make diagnosis
Grade 3: Goiter clearly visible at a distance
Of note: Substernal goiters may not meet typical classification depending on the amount of the goiter present in the neck.
However, goiters which become substernal in their growth pattern have a higher likelihood to become symptomatic . As the goiter grows below the sternal notch and into the thoracic inlet, the trachea and esophagus can become compressed between the sternum and thyroid anteriorly and the cervical and upper thoracic spine posteriorly (see Fig. 2).
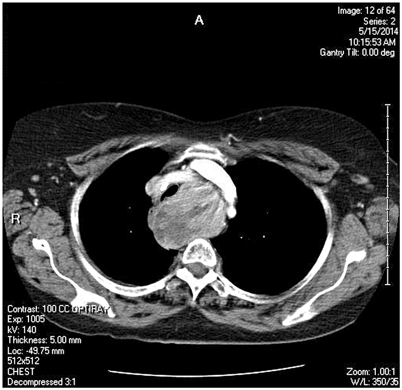

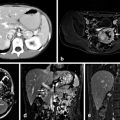
Fig. 2
Substernal goiter leading to significant tracheal deviation and compression with symptomatic dyspnea on exertion
This compression can lead to true dysphagia characterized by difficulty in swallowing solid foods or odynophagia with pain upon swallowing. This should be distinguished from a complaint of a “globus pharyngeus” which implies a sensation of lump in the back of the throat where no lump is actually felt on exam or visualized on inspection or radiographic imaging . The most common causes of globus are gastroesophageal reflux (GERD), sinus drainage, or dis-coordination of the muscles involved in the swallowing mechanism. While patients who have true compressive symptoms or even sleep apnea related to their MNG may benefit clinically from surgery [15, 16], patients with globus sensations should be cautioned that thyroidectomy may or may not improve their symptoms and other evaluations such as laryngoscopy, video endoscopy (esophagogastroduodenoscopy (EGD)), barium swallow, and treatment of other causes as listed above should be considered before undertaking surgery.
Another form of compression results from tracheal compression, which again usually occurs in the setting of a multinodular substernal goiter (SG) . Patients may complain of dyspnea on exertion or positional dyspnea (PD)—worsening shortness of breath when reclined or lying flat. Stang et al. from the University of Pittsburgh provided an evaluation of almost 200 patients with PD in the setting of SG. They demonstrated that SG led to some degree of tracheal compression in 97 % of their patients when evaluated by computed tomography (CT) scan . Improvement in PD after surgical resection was predicted by a gland weight of > 100 g (normal thyroid is approximately 15–20 g) or tracheal compression of > 35 % of tracheal diameter [17] . In the office, the surgeon should ask detailed questions regarding respiratory status both at rest and during exertion. The patient should be questioned about orthopnea and sleeping habits. On physical examination, if the lower extent of the thyroid cannot be palpated during swallowing, an SG should be suspected. Pemberton’s sign [18] can also be a useful physical exam finding to point to a goiter as the cause of compressive symptoms. Named for Dr. Hugh Pemberton who in 1946 described the maneuver , Pemberton’s sign can be used to detect compression at the thoracic inlet from goiter or other anterior mediastinal masses. The patient is asked to extend their arms over their head next to their face which raises the clavicles, posteriorly displaces the upper ribs and narrows the thoracic inlet. If an anterior mediastinal mass is present, the additional compression will result in facial congestion, cyanosis, or respiratory distress in < 1 min.
Patients with subjective complaints of compressive symptoms accompanied by a physical exam suggestive of an SG or positive Pemberton’s sign should undergo a CT scan of the neck and chest to determine the extent of the SG and degree of tracheal compression to aid in surgical and anesthetic planning [19] .
In regard to vocal changes associated with MNG, persistent hoarseness of the voice should prompt preoperative vocal cord assessment. Vocal cord paresis strongly suggests the presence of invasive thyroid malignancy. Persistent vocal changes in the setting of benign MNG are uncommon .
Risk of Malignancy in MNG
The final consideration of surgical intervention in the setting of MNG is the risk of malignancy . A common misconception is that an MNG implies a benign etiology or has a substantially lower risk of malignancy when compared to a solitary nodule. Several independent studies have shown the risk of malignancy in MNG to be 10–31 % [20–22]. A recent large meta-analysis (23,000 patients) review by Brito et al. evaluated the risk of malignancy of single nodule versus MNG [23]. While their overall conclusion was that single nodules had a slightly higher risk of malignancy compared to solitary thyroid nodules, the overall risk of malignancy in surgically resected MNG was 8–15 %.
Thus, the evaluation for cancer in MNG should not differ from solitary nodules. Patients with nodules identified on physical exam or by other radiographic tests such as CT scan, positron emission tomography (PET) scan, and carotid duplex should undergo a dedicated thyroid ultrasound to accurately measure nodules and characterize them to see if they meet American Thyroid Association guidelines for biopsy [24]. Nodules > 1 cm should undergo biopsy. Nodules less than 1 cm with atypical features or in patients with a family history of thyroid cancer or personal history of radiation exposure should be “considered” for biopsy .
Ultrasound may also be very helpful in determining which nodules to biopsy based on ultrasonographic criteria that have been shown to predict a higher incidence of malignancy (see below). Biopsies performed under ultrasonographic guidance by experienced physicians have excellent sensitivity, specificity, and accuracy (95, 87.7, and 89 %, respectively) [25].
Ultrasonographic Features Worrisome for Thyroid Malignancy
1.
Irregular borders
2.
Microcalcifications
3.
Lesions that are taller than they are wide
4.
Hypoechoic lesions
5.
Hypervascular thyroid nodules (central not peripheral pattern)
6.
Abnormal lymph nodes (microcalcifications, loss of fatty hilum)
One question that frequently occurs in the setting of MNG is how many nodules should be biopsied to predict that the remaining nodules are benign. Ultrasonography as mentioned above can guide biopsy of nodules with worrisome features . Thyroid uptake scanning (I-123) has also been used to some extent [26] to find “cold” nodules which have a generally accepted rate of malignancy of 10–20 %. However, this test cannot reliably exclude malignancy in a thyroid nodule. Some texts have advocated simply biopsying the “dominant” or “largest” nodule. Returning to Frates et al. [22], we see that when they biopsied either solitary thyroid nodules or MNG, the rate of cancer in both groups was similar at 14.8 and 14.9 %, respectively. On subgroup analysis of the MNG patients, if they had only biopsied the largest nodule in patients with at least two nodules > 1 cm in size, they would have missed a cancer in the nondominant nodule in 14 % of patients. If the MNG contained at least three nodules ( > 1 cm), biopsy of the dominant lesion would have missed another malignancy in 50 % of patients. Thus, they advocate biopsying up to four nodules in the setting of MNG to exclude a risk of cancer in additional nodules .
Treatment Options for MNG
Medical Treatment
Medical treatment options generally employ either TSH suppression with levothyroxine (LT4) administration or radioactive iodine thyroid ablation . In the setting of hypothyroidism, excess TSH production can result in hyperplasia, nodular growth, and a general increase in goiter size. Here, the use of LT4 to induce a euthyroid state has been clearly documented to decrease goiter size [9, 10]. However, in the circumstance of the euthyroid MNG, simple LT4 administration is unlikely to have any meaningful effect. Therefore, some groups have advocated high-dose LT4 administration in suppressive doses to significantly lower TSH. Moalem and Duh [27] provide an evidence-based review of the literature on its effectiveness. They reviewed two large meta-analyses, including over 13 major studies and nine randomized trials. In 2002, Castro et al. [28] found six randomized trials comprising almost 350 patients who had a benign solitary thyroid nodule treated with high-dose suppressive LT4. And 22 % of patients treated with LT4 had a 50 % decrease in nodule volume compared to only 10 % of patients treated with placebo; however, p values were not significant. In a follow-up study, Sdano et al. [29] expanded on this work by including nine randomized trials (650 patients and some with MNG) . 22 % of patients who underwent suppressive LT4 therapy had a 50 % decrease in nodular size, whereas patients who received no treatment or placebo therapy only had a 10 % rate of significant decrease in nodular volume. These results were statistically significant, but as Moalem et al. indicated, “eight patients (with nodular thyroid disease) would need to be treated in order to have one patient benefit,” from suppressive LT4 therapy. While these data do hold some promise, there is a concern with LT4 medical treatment that should be raised. This treatment method may often be employed in patients who are poor surgical candidates (elderly, cardiac disease, pulmonary disease, etc.). These are the very patients who may also experience complications due to high-dose LT4 treatment, including osteoporosis [30], atrial fibrillation, and other cardiac arrhythmias. Based on these complications, the American Thyroid Association issued a concern regarding long-term treatment of nontoxic MNG with LT4 therapy and stated, that it is, “generally not recommended.” The medical community has also commented that, “as a result of poor efficacy and potential side effects from the induction of subclinical hyperthyroidism,” LT4 treatment of MNG has fallen out of favor [31, 32] . Finally, it must be noted that if LT4 therapy is chosen for the management of MNG, it must be continued indefinitely as it is has been shown that the goiter and nodules may return to their original size within as little as 3 months after discontinuation of suppressive treatment [33].
Another option for the medical treatment of MNG is the use of radioactive iodine (I-131) in ablative doses. This treatment modality is often used for Graves’ disease or for toxic MNG. In addition, it has been successfully used to treat MNG for more than 25 years, where it has been shown to cause a decrease in overall thyroid volume of up to 40 % in the first year and 50–60 % 2 years after treatment [34, 35] . However, its effects are dependent on the ability of the thyroid and its nodules to both uptake and organify iodine. Many nontoxic MNG contain “cold” nonfunctional nodules which can limit the effectiveness of I-131 treatment. Therefore, several groups have employed an off-label use for recombinant human TSH (RhTSH) to increase the uptake of I-131 (radioactive iodine uptake, RAIU) up to two- to threefold [36]. Several trials have demonstrated this improved RAIU with RhTSH pretreatment. These studies have also shown that with RhTSH pretreatment, you can use a decreased dose of I-131, still leading to improved reduction of compressive goiters [37, 38] and even lessen the degree of tracheal compression from SG [39] .
While these results are impressive, the advocates of medical treatment of MNG freely admit that it cannot be used in all circumstances and acknowledge that it has potential side effects and drawbacks. I-131 treatment +/− RhTSH is considered contraindicated for patients with an MNG with suspicion of malignancy, goiter during pregnancy, and in patients with severe tracheal compression and overt symptoms. I-131 can lead to acute swelling of MNG which could worsen tracheal compression or other symptoms. RAI use should also be considered a relative contraindication for young patients with large goiter, in patients requiring rapid decompression of compressive goiter, or if prior I-131 treatment has failed to provide adequate relief of symptoms. Its best use may be for patients with mild to moderate compressive symptoms, elderly patients with comorbid conditions that preclude surgery, or in patients who have undergone prior thyroidectomy in which reoperative surgery is considered too risky. This should only be done once cancer has been excluded by biopsy .
Surgical Management
Surgical Indications
The most clear-cut indication for surgical management of an MNG is for the definitive treatment of thyroid cancer . As mentioned above, the overall risk of malignancy in an MNG ranges from 10 to 31 %; the majority of these, approximately 60 %, are papillary thyroid carcinomas [20–22, 40] which are often multifocal. Thyroidectomy for lesions “suspicious for malignancy,” including Bethesda class 3–5 lesions [41] (BC 3: atypia of undetermined significance, BC 4: follicular/Hurthle neoplasms, and BC 5: suspicious for papillary thyroid carcinoma), can give definitive pathologic diagnosis, eliminate the need for long-term ultrasonographic surveillance, and potentially allow for surgical cure of cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree