© Springer Science+Business Media New York 2015
Terry F. Davies (ed.)A Case-Based Guide to Clinical Endocrinology10.1007/978-1-4939-2059-4_3838. Getting Pregnant with PCOS
(1)
George Washington University School of Medicine and Health Sciences, 2150 Pennsylvania Avenue, Washington, DC 20037, USA
Keywords
Polycystic ovarian syndrome (PCOS)PregnancyOvulatory disturbanceNIH criteriaOligo-ovulationHyperandrogenism (clinical or biochemical) or sonographic findingsHirsutismPregnancyAnovulationCancerMetabolicCardiacObjectives
1.
To describe the diagnostic features of polycystic ovarian syndrome.
2.
To apply current therapies in order to induce ovulation and/or achieve pregnancy in women with polycystic ovarian syndrome.
Case Description
A 36-year-old married gravida 0 sees her OB/GYN after stopping oral contraceptive pills (OCPs) in order to get pregnant. She started using OCPs while in college to help with acne. Her weight, since her wedding 5 years ago, has steadily increased by 4–5 pounds per year. While her menstrual cycles on OCPs were regular, every 25 days since OCP discontinuation, her cycles became irregular coming every 6–9 weeks lasting 8 days. Her periods are free of cramps but can be heavy warranting a change in sanitary napkin every hour for the first day. She is otherwise healthy without medical problems and sees her gynecologist on an annual basis. She has never had an abnormal PAP smear and takes no medications other than a daily multivitamin. She is 5′7″ tall and weighs 180 pounds (BMI 28.2 kg/m2). Her physical examination is notable for a blood pressure of 134/88, increased hair growth on her chin and posterior thighs, and she has a skin tag in her left axilla. The following test results are reported in a workup for anovulation: hCG <1.0 mIU/ml; FSH 5.6 mIU/ml; TSH 1.480 mIU/L; FT4 1.21 ng/dL; Prolactin 9.3 ng/mL; 17-OHP 120 ng/dl; DHEAS 240 ug/dl; Total Testosterone 55 ng/dl; HgbA1c 6.0 %; fasting glucose 88 mg/dl; fasting insulin 21 uU/ml; transvaginal ultrasound demonstrates an endometrial lining of 1.2 cm and bilateral ovarian diffuse enlargement with multiple peripheral cysts in a “string of pearls” configuration.
Introduction
Affecting between 6 % and 15 % of women, polycystic ovarian syndrome (PCOS) is the most common female endocrine disorder [1]. Commonly, PCOS presents during adolescence and continues throughout a woman’s reproductive life [2]. The clinical features typically include a combination of ovulatory disturbance and hyperandrogenism with or without characteristic polycystic ovaries [3]. There remains no unifying theory as to the pathophysiologic basis of PCOS and the phenotype can be highly variable. Two distinct conventions regarding the requisite diagnostic findings when considering PCOS remain [3, 4]. The NIH criteria include a combination of ovulatory disturbance and hyperandrogenism in the absence of other conditions that share clinical findings. Because of the marked heterogeneity amongst PCOS presentations, the Rotterdam criteria included any two of the three classic features: oligo-ovulation, hyperandrogenism (clinical or biochemical) or sonographic findings [3]. Management of PCOS involves an understanding of the various aspects of women’s health including hirsutism, pregnancy, anovulation, cancer, metabolic, and cardiac well being. This patient’s history of hyperandrogenism began shortly after adolescence and, while controlled by OCPs, returned after she discontinued OCPs. This presentation will focus on diagnosing PCOS and interventions aimed at achieving pregnancy.
Clinical Features
Typically, PCOS is associated with of hyperandrogenism, the most distinctive feature of which is hirsutism [2, 5]. Hirsutism can be mild to severe and in patients with PCOS it is usually of a gradual onset [6]. Rapid onset of severe hirsutism with thick pigmented hair is suggestive of a neoplastic source of androgens [7, 8]. In PCOS, the areas most commonly involved include the sides of the face, chin, upper lip, neck, posterior thigh, and the upper abdomen with extension from the pubic escutcheon. In severe cases, sexual hair can be seen on the chest and may be associated with temporal balding [6].
Menstrual bleeding is usually irregular and may be absent. In many cases, the menstrual pattern never becomes regular and may transition from irregular cycles to intervals of amenorrhea. While nearly 20 % of women with PCOS have no menses, those with very heavy periods should be followed for endometrial hyperplasia or carcinoma [7].
Roughly half of women with PCOS are obese [9]. Obese women with PCOS commonly have an increased waist-to-hip ration and android fat pattern. Such increased visceral fat is commonly seen in individuals with insulin resistance and may serve as a marker of metabolic dysfunction [7]. Both thin and obese women with PCOS commonly exhibit insulin resistance [10–12]. Insulin resistance may lead to a reduction in sex hormone-binding globulin (SHBG) leading to more bioavailable androgens for receptor binding [12, 13]. High concentrations of peripheral insulin can lead to an insulin action on the insulin-like growth factor I (IGF-I) receptor in the ovary. IGF-I receptor activation leads to enhanced ovarian testosterone production. Women with insulin resistance may demonstrate hyperpigmentation in areas of skin folds like the axillae and groin (acanthosis nigricans) and they can be prone to developing skin tags [7, 14].
Women with PCOS are at an increased risk for both gestational and nongestational diabetes [15, 16]. By improving glucose metabolism and amelioration of insulin resistance, androgen levels can be reduced and ovulation restored in some patients with PCOS. This can be accomplished with weight loss, insulin lowering medications, or a combination of the two [15, 17, 18].
Women with PCOS demonstrate arrested development of antral follicles. Such cysts persist for a period of time only to undergo atresia and replacement by similar peripherally situated mid-antral cysts. The cysts are nested in a hyperplastic stroma that exhibits enhanced androgen production [7]. The morphologic characteristics of the PCOS ovary seem to derive from enhanced ovarian androgen exposure. Therefore the PCOS ovary both reflects and creates a hyperandrogenic environment [5]. Peripherally, androgens are converted to estrogens by aromatase expressing adipose tissue. Chronic exposure to unopposed estrogens results in multiple physiologic changes [5].
Polycystic ovarian syndrome is notable for an alteration in LH secretion. Women with PCOS demonstrate both increased pituitary LH pulse frequency and amplitude [19]. The underlying cause of this pituitary malfunction is not known, but it may be partially driven by chronic exposure of the pituitary gonadotroph to elevated estrogen levels [7]. Such exposure may alter the gonadotroph’s sensitivity to GnRH leading to increased LH pulse amplitude, frequency or both. Increased pituitary LH release leads to enhanced ovarian androgen production further exacerbating both the local follicular dysfunction with follicular arrest and the peripheral hormonal environment with chronically elevated peripheral estrogen levels [19].
Unopposed estrogen exposure leads to chronic proliferation of the endometrial lining with the potential for hyperplasia and carcinoma. Endometrial carcinoma has been reported in young women with ovulatory disturbances and young women with endometrial cancer have been noted to have a high frequency of anovulatory menstrual irregularities. Polycystic ovarian syndrome does not appear to increase the risk for either breast or ovarian cancer [7].
The combination of obesity, hyperandrogenism and insulin resistance increases the potential that women with PCOS develop heart disease [20]. It is unknown if PCOS is an independent risk factor for heart disease. Women with PCOS exhibit dyslipidemia with high levels of LDL and triglycerides and low levels of HDL. The combined effects of obesity, dyslipidemia, insulin resistance, and hyperandrogenemia increase the potential for plaque formation on coronary vessels. Hypertension maybe more common among PCOS individuals further exacerbating cardiac risk. However, whether hypertension is independently associated with PCOS or if it is related to obesity remains unclear [20].
Diagnosing PCOS
Central to the diagnosis of PCOS is the exclusion of other conditions that share clinical findings. Congenital adrenal hyperplasia (CAH) leads to increased serum androgens as a result of a relative block in cortisol production. 21 hydroxylase deficiency can mimic PCOS. It results in an increased production of cortisol precursors which include androgens. One can rule out CAH prior to diagnosing PCOS by assessing serum 17-OHP levels. Women with CAH exhibit an elevation in 17-OHP, a cortisol precursor, and normal 17-OHP levels (<200) exclude CAH from the differential diagnosis [3].
It is also important to exclude androgen producing neoplasms as the cause of the hyperandrogenemia. Androgen producing neoplasms usually exhibit rapid acuity and markedly elevated androgens. Sudden onset or severely virilizing symptoms suggest a neoplastic process. Measurement of serum total testosterone and DHEAS can both establish the presence of an androgen producing tumor and localize its etiology. Testosterone is made both by the ovaries and adrenal glands while DHEAS is made exclusively by the adrenal glands. The presence of markedly elevated testosterone and normal DHEAS levels suggest an ovarian etiology while elevations in both reveal an adrenal source.
Excluding nonovarian causes of ovulatory dysfunction is also critical in establishing PCOS as a diagnosis. Normal FSH levels rule out ovarian failure as a cause of anovulation. Both thyroid dysfunction and hyperprolactinemia can be easily excluded with a routine blood draw.
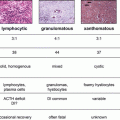
Finally pelvic sonography can be helpful by assessing for the presence of multiple peripherally located small follicular cysts. These must be differentiated from ovaries with multiple nonperipherally distributed follicular cysts. The latter is a normal variant seen in the youthful fertile ovary [3, 8]. In PCOS, the ovarian volume is increased. Furthermore, a thickened endometrial lining can be suggestive of endometrial hyperplasia.
Hyperandrogenemia leads to continuous peripheral conversion of testosterone to estrogen by aromatase containing tissue including muscle and adipose. The general lack of ovulation translates to a lack of progesterone production and unopposed estrogen action on the endometrium. This increases the risks of meno-metrorrhagia, endometrial hyperplasia and endometrial adenocarcinoma. In patients with irregular menses and a thickened endometrial stripe (>5 mm), endometrial sampling followed by histological assessment is warranted.
PCOS and Fertility
Owing to reduced ovulation, women with PCOS demonstrate reduced fertility. Furthermore, an increase in early fetal wastage is noted in women with PCOS [15, 17]. The increased miscarriage rate may be related to obesity. It has been suggested that both hyperandrogenemia and hyperinsulinemia may contribute to fetal wastage, however the exact mechanism by which these effect early pregnancy remains unclear [9].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree