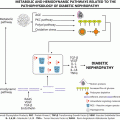
Fig. 28.1
The Pedersen Hypothesis. “Maternal hyperglycemia begets foetal macrosomia” Modified by Freinkel to include nutrients other than glucose (lipids and amino acids)
Prior diagnostic pathways and nomenclature for diagnosis of GDM have been heterogeneous. However, the recently published results of the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study [6] and subsequent consensus development process auspiced by the International Association of Diabetes in Pregnancy Study Groups (IADPSG) [7] have led to a clearer and more uniform approach, grounded primarily in the risk of adverse pregnancy outcomes associated with mild hyperglycaemia in pregnancy. The IADPSG criteria [7] will be the primary point of reference for diagnosis of GDM in this paper.
The HAPO study [6] demonstrated a continuous and essentially linear relationship between maternal glycaemia, measured during a three sample (fasting, 1 h, 2 h) 75 g OGTT performed between 24 and 32 weeks gestation and a series of clinically important pregnancy complications, including excessive foetal growth (both large for gestational age babies and those with excess body fat), risk of neonatal hyperinsulinemia, primary caesarean section and risk of pre-eclampsia. The independent associations of glucose with these outcomes persisted after adjustment for multiple potential confounders, including maternal Body Mass Index (BMI) [6, 8]. HAPO also considered the potential role of glycosylated haemoglobin (HbA1c) in the detection of GDM, but concluded that HbA1c was not sufficiently discriminatory to be of value in this setting. Further, HAPO confirmed the Pedersen hypothesis [9] by clearly demonstrating the association between even mild degrees of maternal hyperglycaemia and the foetal consequences of LGA, increased adiposity and hyperinsulinemia.
Importantly, the HAPO study demonstrated no threshold for glucose associations with adverse outcomes, suggesting that new diagnostic criteria for GDM would need to be developed through a consensus process, a conclusion reached one and a half decades previously by Sacks et al. [10]. The consensus process auspiced by IADPSG subsequently developed a two stage protocol for diagnosis of GDM and related disorders, summarized in Fig. 28.2. The recommended diagnostic values for GDM were determined by consensus. They were based on the risks of foetal outcomes related to maternal glycaemia, specifically LGA, neonatal adiposity (body fat > 90th centile) and neonatal hyperinsulinemia. The diagnostic glucose thresholds chosen (fasting, 1 h or 2 h at 75 g OGTT) were those associated in continuous statistical models with Odds Ratios of 1.75 compared to the HAPO cohort mean for the three outcomes mentioned above, after extensive adjustment for other confounders [7].
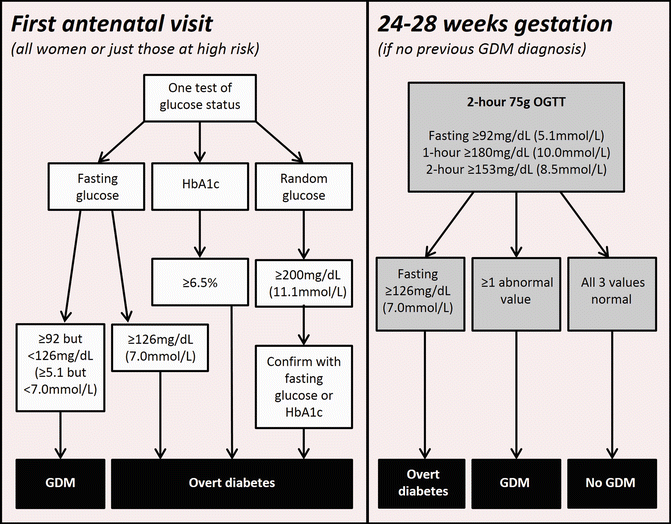

Fig. 28.2
IADPSG recommended diagnostic pathway for overt diabetes in pregnancy and gestational diabetes
In recognition of the increasing prevalence of pre-pregnancy diabetes (principally Type 2 diabetes) and obesity in women of reproductive age across many populations, IADPSG recommended early pregnancy testing for undiagnosed, severe degrees of hyperglycaemia in early pregnancy. Implementation of early pregnancy testing on a universal or selective (risk factor based) basis was left open in the IADPSG recommendations due to the lack of firm data in this field and the widely varying diabetes prevalences in different populations around the world. The IADPSG Guidelines recommend that testing be undertaken using any of the following: fasting glucose, HbA1c or random glucose (with confirmation) and that the results should be assessed using existing non-pregnancy-specific diagnostic criteria (see Fig. 28.2).
Those women with results consistent with diabetes outside pregnancy should be labelled as having “Overt Diabetes” in view of their more severe hyperglycaemia. Further, hyperglycaemia of this degree, if detected in early pregnancy, is likely to have antedated the diagnosis of pregnancy. Clinically, these women have been shown to have high rates of established microvascular diabetes complications [11] and thus demand urgent attention, both for their pregnancy care and for management of their diabetes and associated complications.
The IADPSG recommends that all women not previously identified as having abnormal glucose metabolism should undergo a formal 75 g OGTT at 24–28 weeks’ gestation. Non-fasting glucose challenge tests, favoured in some previous diagnostic algorithms, are not recommended due to limited sensitivity [12] and lack of clear associations with pregnancy outcomes. Following the IADPSG criteria, GDM is diagnosed if any one of the three relevant glucose values (fasting, 1 or 2 h post 75 g glucose load) equal or exceed the thresholds noted in Fig. 28.1. If a fasting test is performed in early pregnancy, some women may be diagnosed as GDM at this stage. Otherwise, the diagnosis would generally be made at 24–28 weeks.
Given the fine distinctions to be drawn between normal and abnormal results, standardization and calibration of glucose measurements at the OGTT and at other testing points in pregnancy is critical. Venous plasma glucose should be used for all measures and laboratories should pay careful attention to methodology and quality control [7].
Differential Diagnosis/Other Considerations
Since GDM is most commonly an asymptomatic condition diagnosed on the basis of glucose concentrations during the exogenous stress of an OGTT, differential diagnosis is not generally an important clinical consideration. However, it is important to consider other aspects of the condition including [1] other specific forms of abnormal glucose metabolism which may present as GDM and [2] important maternal and pregnancy factors which predispose to GDM.
Monogenic causes of diabetes are increasingly recognized and though they tend to comprise only a small proportion of cases, they may be first detected in pregnancy due to increased glucose testing. Autosomal mutations causing diabetes are commonly grouped under the heading of Maturity Onset Diabetes of the Young (MODY). Collectively, MODY variants may account for up to 10 % of cases of GDM [13].
The example of glucokinase gene (GCK) mutations causing MODY2 [14] is particularly instructive. GCK mutations in the mother are associated with mild fasting hyperglycaemia, generally without other abnormalities on the oral glucose tolerance test. If the foetus carries the same mutation, birthweight tends to be normal as both mother and foetus are adapted to “sensing” glucose at the same level. By contrast, if the mother carries the GCK mutation (and therefore is mildly hyperglycaemic) and the foetus is normal, birthweight is increased. Conversely, if the mother is normal and the foetus carries the GCK mutation, birthweight is reduced. This elegant experiment of nature demonstrates the potential importance of both genes and environment in determining foetal growth.
Autoimmune diabetes, including early Type 1 diabetes and Latent Autoimmune Diabetes of Adult Life (LADA) may also be detected due to glucose testing in pregnancy. The definitive diagnosis is not always clear in this instance. In a recent case series from France, this form of diabetes, described as “Type 1 diabetes masquerading as GDM” was associated with pregnancy complications [15]. Prevalence of GAD or islet cell antibodies has been reported to be as high as 6 % in some GDM cohorts [16] and is associated with a higher incidence of progression to overt diabetes post-partum.
The use of clinical risk factors for prediction of GDM is of limited utility and this forms one argument in favour of universal diagnostic testing in pregnancy. Increased placental size, as seen in multiple pregnancies, is associated with a higher risk of GDM in some studies [17]. Whilst a number of demographic and anthropometric factors, such as maternal age, ethnicity, body mass index and previous history of GDM or macrosomia are associated with higher risk of GDM, no one factor or combination of factors offers sufficient discriminatory power to obviate biochemical assessment.
Reported recurrence rates of GDM in subsequent pregnancies vary from 36 to 69 % [18]. Recurrence risk appears higher in the presence of maternal obesity, early diagnosis of GDM in the index pregnancy and excessive inter pregnancy weight gain. It remains unclear why recurrence rates for GDM are not higher than observed, given that women are by definition older and frequently heavier at the time of subsequent pregnancies. This observation does suggest that variability in the interaction between a woman and a particular foetus (or feto placental unit) may play a role in the aetiology of GDM.
Current and Future Therapies
Current therapy for GDM is largely “glucocentric”, with the major therapeutic goal being achievement of glucose levels as close to normal pregnancy values as possible. Our discussion will include the current definition of normoglycaemia in pregnancy, evidence favouring treatment, available therapeutic modalities, recent evidence for the use of ultrasound estimation of foetal growth to guide the intensity of treatment and decisions regarding the mode and timing of delivery. Post-partum follow-up for the woman with GDM will also be addressed.
Normoglycaemia in Pregnancy
The definition of normal pregnancy glucose levels, both fasting and in the postprandial state, has been investigated by a variety of methods, including detailed hospital inpatient studies, ambulatory studies using capillary glucose meters and continuous glucose monitoring (CGMS) over many years. A comprehensive analysis of this literature was published by Hernandez et al. in 2011 [19] and concluded that mean glucose levels in normal weight pregnant women were lower than previously described (Mean ± SD Fasting 71 ± 8 mg/dL or 3.9 ± 0.4 mmol/L; 1 h post meal 109 ± 10 mg/dL or 6.1 ± 0.6 mmol/L; 2 h post meal 99 ± 10 mg/dL or 5.5 ± 0.6 mmol/L). The commonly used statistical upper limits of these observations (Mean + 2 SD) are also substantially lower than current, largely empirical, glucose treatment targets advocated in GDM [20–25] (Table 28.1) The determination of optimal glucose targets remains contentious, although the current recommendations of major professional bodies are largely congruent. The values mentioned in Table 28.1 are the maximal glucose values in the fasting and post-prandial states considered acceptable by the relevant organizations, or reported as treatment targets by the randomized controlled trials. The abbreviations and data sources for Table 28.1 are as follows: ADA—American Diabetes Association [20, 22]; ADIPS—Australasian Diabetes in Pregnancy Society [21]: ACOG—American College of Obstetricians and Gynecologists [22]; NICE—National Institute of Clinical Excellence (UK) [22]: DIPSI—Diabetes in Pregnancy Society India [23]: CDA—Canadian Diabetes Association [24, 25]; ACHOIS—Australian Carbohydrate Intolerance Study [26]; MFMN—Maternal Foetal Medicine Networks study [27]
Table 28.1
Glucose targets recommended by major national organizations in gestational diabetes or reported in major randomized controlled trials
Organization | Fasting (≤) | 1 h post meal (≤) | 2 h post meal (≤) |
|---|---|---|---|
ADA | 95 mg/dL 5.3 mmol/L | 140 mg/dL 7.8 mmol/L | 120 mg/dL 6.7 mmol/L |
ADIPS | 99 mg/dL 5.5 mmol/L | 144 mg/dL 8.0 mmol/L | 126 mg/dL 7.0 mmol/L |
ACOG | 95 mg/dL 5.3 mmol/L | 130 mg/dL 7.2 mmol/L | |
NICE | 106 mg/dL 5.9 mmol/L | 140 mg/dL 7.8 mmol/L | |
DIPSI | 90 mg/dL 5.0 mmol/L | 120 mg/dL 6.7 mmol/L | |
CDA | 95 mg/dL 5.3 mmol/L | 140 mg/dL 7.8 mmol/L | 120 mg/dL 6.7 mmol/L |
Trial | |||
ACHOIS | 99 mg/dL 5.5 mmol/L | 126 mg/dL 7.0 mmol/L | |
MFMN | 95 mg/dL 5.3 mmol/L | 120 mg/dL 6.7 mmol/L |
Evidence for Treatment of GDM
Although the clinical importance of treating GDM was hotly debated for many years, two recent randomized controlled trials have now been conducted, with largely congruent results. The ACHOIS study [26], conducted principally in Australia, randomized 490 women to an intervention including dietary advice, home blood glucose monitoring and insulin therapy as required and compared them to 510 women assigned to standard care. The primary composite outcome of serious perinatal complications (death, shoulder dystocia, bone fracture or nerve palsy) was reduced from 4 to 1 % (p = 0.01) with intervention. Only 20 % of women required adjunctive insulin treatment. The rates of LGA and macrosomia (defined as birthweight ≥ 4,000 g) were also reduced, with no increase in small for gestational age (SGA) babies. Quality of life was improved with treatment and hypertensive complications of pregnancy were less frequent. Treated women had higher rates of induction of labour, but similar rates of caesarean delivery. Admission of neonates to the neonatal nursery was more frequent with intervention, possibly related to hospital policies.
The USA based Maternal-Foetal Medicine Units Network trial of the treatment of mild GDM [27] randomly assigned 485 women to intervention vs. 473 assigned to standard care. This study excluded women with fasting glucose ≥ 95 mg/dL, but required them to have 2 / 3 other OGTT values in excess of ADA thresholds for the diagnosis of gestational diabetes [22]. The composite outcome used in this trial included both perinatal mortality and neonatal outcomes associated with maternal hyperglycaemia (hypoglycaemia, hyperbilirubinemia, hyperinsulinemia and birth trauma. Fully 92 % of treated women were managed by lifestyle interventions, with only37/485 or 7.6 % needing adjunctive insulin therapy. As with the ACHOIS study, the rates of LGA and macrosomia were reduced with treatment as was neonatal fat mass. Hypertensive disorders of pregnancy, caesarean section, maternal weight gain from enrolment and shoulder dystocia were all reduced by active intervention. The composite neonatal outcome did not differ between the treatment groups.
Considering these two trials together, we conclude that active treatment of women with GDM reduces serious pregnancy complications including hypertensive disorders of pregnancy and excessive foetal growth and its consequences. Thus, after many years of debate, active treatment of GDM appears justified.
Current Therapy of GDM
Lifestyle modification, including medical nutrition therapy and encouragement of physical activity, forms the primary mode of therapy for GDM. As noted above, such therapy proved sufficient in 80–90 % of women enrolled in the two major randomized trials. The American Dietetic Association evidence based nutrition practice guidelines for GDM [28] provide a sound overall framework for nutritional interventions. They advocate review by a registered dietitian within 1 week of GDM diagnosis and a minimum of two follow up visits. Whilst dietary recommendations are individualized, the general guidance favours mild energy restriction to approximately 70 % of recommended daily intake for overweight/obese women and reduction of total carbohydrate intake to less than 45 % of total caloric intake. Other recent studies have specifically examined the role of glycaemic index in nutritional therapy for GDM [29] and have suggested that this may enhance the effects of standard treatment and assist some women in meeting glycaemic targets and obviating the need for pharmacotherapy, although overall pregnancy outcomes have been reported as similar to those achieved with a high-fibre diet [30].
Insulin therapy remains the cornerstone of treatment for women who fail to meet glycaemic goals after lifestyle modification. The glucose targets currently recommended by various international organizations and those used in the two major randomized trials are listed in Table 28.1. The precise insulin regimen used for an individual woman depends on the pattern of elevated glucose readings seen on home glucose monitoring. Women with predominant fasting hyperglycaemia may respond well to a single evening injection of intermediate or long-acting insulin. Those with predominant elevation of post-prandial glucose are generally treated with soluble insulin or a rapid-acting analogue insulin prior to meals. Women with a mixed pattern of elevated glucose readings may also be treated with premixed insulins, generally twice daily at breakfast and dinner time. A recent detailed review [31] has concluded that there is minimal evidence available to choose between specific insulin regimens.
Newer Therapeutic Options
Oral hypoglycaemic agents, principally glibenclamide (glyburide in the USA) and metformin, have also been trialled in gestational diabetes. The first major randomized controlled trial of glyburide, conducted by Langer and colleagues [32] reported equivalent glucose control and no substantial differences in outcomes in women treated with glyburide as compared to insulin after failure of lifestyle management. Langer’s study was not powered to examine major pregnancy outcomes. Subsequently, a systematic review of this and subsequent randomized trials [33] reported no differences in terms of glycaemic control or pregnancy outcomes when comparing glyburide and insulin therapy. However a large recent (non-randomized) cohort report of 10,682 women from the “Sweet Success” programme in California reported increased rates of LGA and neonatal intensive care unit admission in those women treated with glyburide [34].
Metformin has also been evaluated in one large [35] and two smaller randomized trials [36, 37]. These studies included women who failed to meet glycaemic targets after lifestyle interventions for GDM. They demonstrated very similar glucose control and pregnancy outcomes with metformin therapy when compared to standard insulin treatment. The rate of supplemental insulin therapy was high overall—46 % in the MiG study and 32 % in the Finnish report but surprisingly no patients in the study from New Mexico USA required supplemental insulin therapy. Patient preferences were clearly in favour of metformin therapy.
Uncontrolled reports suggested that metformin reduces pregnancy complications in women with polycystic ovarian syndrome treated during pregnancy [38–40], but a recent randomized trial failed to confirm any benefit [41].
Two randomized trials [42, 43] comparing metformin with glyburide/glibenclamide in the treatment of GDM have shown somewhat discordant results. In a report from Brazil [42, 44] Silva et al. reported comparable efficacy between these two medications, with supplemental insulin required in around 25 % of each group. In contrast, Moore et al. [43] reported that 35 % of metformin treated women required supplemental insulin as compared to 16 % of those treated with glyburide.
The use of oral hypoglycaemic agents in the treatment of GDM varies widely on a country by country basis, with glyburide favoured in the USA [45], whilst metformin is recommended more widely in the United Kingdom and Australasia [46–48]. Some practitioners remain concerned by the possibility of adverse foetal effects of oral drugs. Glyburide has been reported only at very low levels in cord blood, due to limited transplacental passage and active counter transport [49, 50]. Metformin crosses the placenta readily [51–53] but has shown no adverse foetal or early childhood effects to date [54, 55].
As noted above, many of the adverse effects of GDM relate to excessive foetal growth, driven by nutrient excess and consequent foetal hyperinsulinism as outlined in the Pederson hypothesis [9] and its subsequent modification by Freinkel and Metzger [5]. These have also been presented in Fig. 28.1. Direct assessment of amniotic fluid insulin concentrations, pioneered by Weiss et al. [56] demonstrated that foetal hyperinsulinemia was associated with increased risks of pregnancy complications. In turn, Schafer Graf et al. [57] noted that foetal abdominal circumference (AC) on ultrasound could be used to non-invasively predict foetal hyperinsulinism and identify those babies most at risk of diabetic fetopathy.
Subsequently, an alternative approach to treatment of GDM, based on ultrasound assessment of foetal growth, in particular foetal AC, has been proposed and evaluated in four randomized studies [58–62]. According to this approach, summarized by Kjos et al. [60], glucose lowering treatment may be intensified in those pregnancies where the foetus shows evidence of accelerated growth, generally determined by foetal AC > 75th centile for gestational age on ultrasonographic assessment. These protocols suggest that “low risk” pregnancies, as defined by normal (<75th centile) foetal AC, may require less stringent glycaemic control and conversely that detection of an increased AC should lead to intensification of glucose lowering therapy.
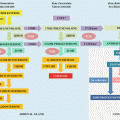
A summary of the traditional (glucocentric) and alternative (USS guided) pathways for GDM therapy is presented in Fig. 28.3.
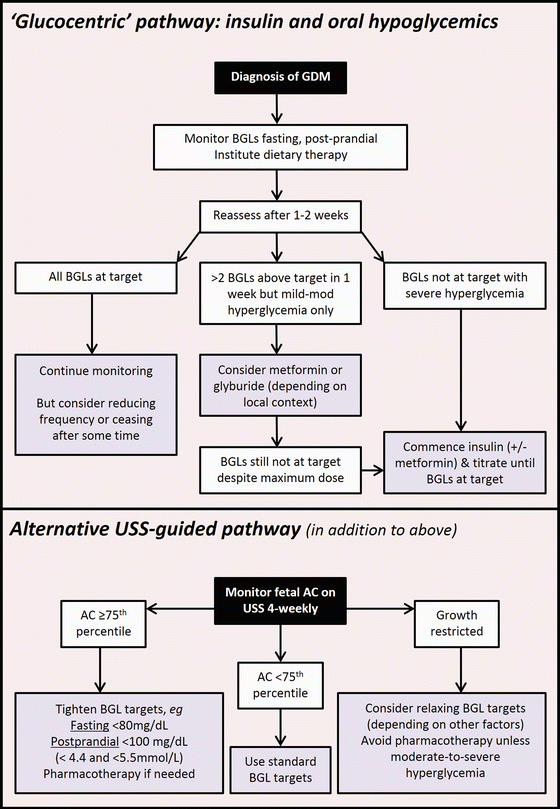

Fig. 28.3
Treatment pathways for GDM—“glucocentric” pathway including treatment with insulin and oral hypoglycemic agents and alternative “USS guided” pathway. For details see text
Foetal Surveillance and Timing of Delivery
Despite a lack of high-level evidence of definite benefit, foetal ultrasound is frequently used to assess foetal well bring and to estimate foetal weight to assist in determining the timing and mode of delivery. Most units have extrapolated their practices in this area from those developed for pre-gestational or pre-existing diabetes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree