Abbreviations
- ADA American Diabetes Association
- BG Blood glucose
- BMD Bone mineral density
- BMI Body mass index
- CHF Congestive heart failure
- CRH Corticotropin-releasing hormone
- CVD Cardiovascular disease
- DHEA Dehydroepiandrosterone
- DPP IV Dipeptidyl peptidase IV
- FFA Free fatty acids
- GFR Glomerular filtration rate
- GIP Glucose insulinotropic peptide
- GLP1 Glucagon-like peptide 1
- GLUT Glucose transporter
- HHNS Hyperglycemic hyperosmolar nonketotic syndrome
- IGT Impaired glucose tolerance
- LH Luteinizing hormone
- LHRH Luteinizing hormone–releasing hormone
- NHANES National Health and Nutrition Examination Survey
- NPH Neutral protamine Hagedorn
- NSAIDS Nonsteroidal anti-inflammatory drugs
- OGTT Oral glucose tolerance test
- PTH Parathyroid hormone
- RANK-L Receptor activator of nuclear factor kappa B ligand
- SERM Selective estrogen receptor modulator
- SIADH Syndrome of inappropriate antidiuretic hormone
- T3 Triiodothyronine
- T4 Thyroxine
- TLI Therapeutic lifestyle intervention
- TSH Thyroid-stimulating hormone
- UKPDS United Kingdom Prospective Diabetes Study
Geriatric Endocrinology: Introduction
Individuals over age 65 comprise the fastest-growing segment of the United States population; each day this group increases by more than 1000 people. This increase has led to a remarkable situation—of all the people who have ever lived to the age of 65, more than two-thirds are still alive. Thus, it is becoming increasingly important for the endocrinologist to understand how endocrine physiology and disease may differ in the elderly.
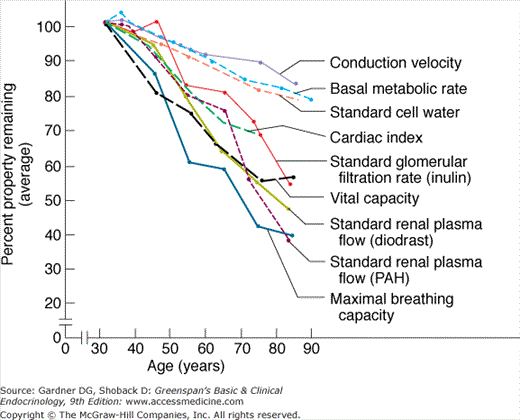
Before considering specific endocrinologic conditions in the elderly, however, it is worthwhile to review some general principles that account for many of the age-related changes in disease presentation in the elderly. First, aging itself—in the absence of disease—is associated with only a gradual and linear decline in the physiologic reserve of each organ system (Figure 23–1). Because the reserve capacity of each system is substantial, age-related declines have little effect on baseline function and do not significantly interfere with the individual’s response to stress until the eighth or ninth decade. Second, because each organ system’s function declines at a different physiologic rate and because 75% of the elderly have at least one disease, endocrine dysfunction in the elderly often presents disparately, with initial symptoms derived from the most compromised organ system. For example, hyperthyroidism in an elderly patient with preexisting coronary and conduction system disease may present with atrial fibrillation and a slow ventricular response, whereas in another equally hyperthyroid patient with a prior stroke, it may present with confusion or depression; neither patient may tolerate hyperthyroidism long enough for the classic thyroid-related manifestations (eg, goiter) to become apparent. Third, elderly patients often have multiple diseases and take many medications that may mimic or mask the usual presentation of endocrine disease.
Thyroid Function and Disease
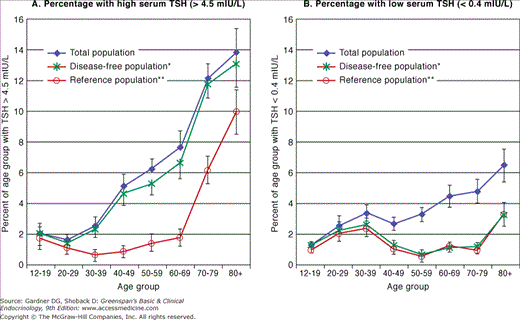
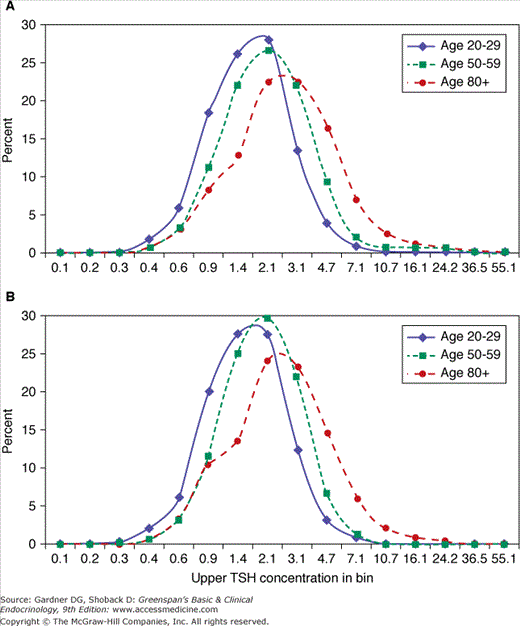
The prevalence of thyroid disease in the elderly is approximately twice that in younger individuals, with hypothyroidism ranging from 2% to 7% and hyperthyroidism affecting up to 2% of older individuals. The Whickham Survey of 21,000 adults in Great Britain and its follow-up study, conducted between 1972 and 1993, reported that the incidence of overt hypothyroidism was increased 10-fold in women age 75 and older when compared to women in their twenties. In the NHANES 1999 to 2002 survey (N=4392) the overall prevalence of hypothyroidism was 3.7% (includes overt and mild) including a prevalence of 5.5% for individuals age 50 to 79 years and 12.1% for those age 80 and older. Individuals age 80 and older had five times greater probability for developing hypothyroidism (OR=5.0, p =0.0002) compared to those 12 to 49 years of age. In addition, some studies suggest that up to 9% of hospitalized elderly patients have overt thyroid disease. Subclinical hypothyroidism—normal serum levels of thyroid hormones (thyroxine, T4; triiodothyronine, T3) with an elevated level of thyroid-stimulating hormone (thyrotropin) (TSH)—is more prevalent, with estimates of 6% to 13% in the elderly (Figure 23–2), and is higher in women than in men. The NHANES III data (N=16,533) suggests that the distribution of TSH shifts to a higher concentration with age and therefore the prevalence of subclinical hypothyroidism may be overshadowed (see Figure 23–3). In the elderly, the progression from subclinical to overt hypothyroidism is roughly 2% to 5% per year. Subclinical hyperthyroidism—normal serum thyroxine with suppressed serum TSH levels—may be found in approximately 2% of elderly subjects (Figure 23–2). The rate of progression to overt hyperthyroidism is less clear. Finally, the overall prevalence of thyroid hormone use in older adults is approximately 7% (10% in women and 2% in men).
Figure 23–2
Percentage with high or low serum thyroid-stimulating hormone (TSH) in the total US population, the disease-free (excludes those people who have reported having thyroid disease, goiter, or taking thyroid medications*) and reference population (excludes those people who reported having thyroid disease, goiter, or taking thyroid medications and those who do not have risk factors such as pregnancy or taking estrogen, androgens, or lithium and who are without the presence of thyroid antibodies or biochemical evidence of hypothyroidism or hyperthyroidism**) by age. A: High serum TSH. In the disease-free population, the percentage of people with high serum TSH concentration (>4.5 μIU/mL) is slightly lower than that of the total population but significantly higher than the percentage in the reference population. B: Low serum TSH. The percentage of people with low serum TSH in the disease-free population, on the other hand, is significantly lower than the percentage in the total population and similar to the pattern seen in the reference population. Note the elevated prevalence of low TSH in the populations without thyroid disease or risk factors at ages 20 to 39 years and again after age 79 years. The higher prevalence of individuals with low TSH or high TSH in the total population is related directly to the people reporting thyroid disease, goiter, or taking thyroid medication and probably reflects inadequate management of clinical thyroid disease. (Reproduced, with permission, from Hollowell JG, et al. Serum TSH, T4, and thyroid antibodies in the US population [1988 to 1994]: National Health and Nutrition Examination Survey [NHANES III]. J Clin Endocrinol Metab. 2002;87:489. Copyright 2002 by The Endocrine Society.)
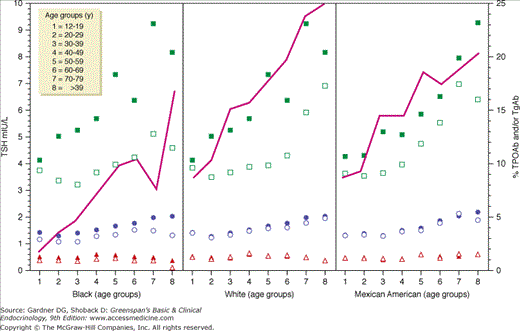
There are few major age-related changes in the physiology of the hypothalamic-pituitary-thyroid axis (Chapters 4 and 7). There are slight age-related increases in serum TSH levels (see Figure 23–3) and decreases in total T4. TSH release remains pulsatile, although the nocturnal rise in serum TSH appears to be blunted with age. There is a slight age-related decline in serum T3, but values usually remain within normal limits. The effect of age on the release of TSH by thyrotropin-releasing hormone is less clear, but most recent studies show little clinically relevant change in either sex. The 24-hour radioiodine uptake is also not significantly altered with age. Thyroid antibodies are common in older women (prevalence up to 25%, Figure 23–4), but their presence does not serve as a specific screening test for thyroid disease. The presence of thyroid antibodies (occult thyroid dysfunction) may skew the upper limit of TSH in the African American, Caucasian, and Mexican American ethnic groups (see Figure 23–4).
Figure 23–4
The panels show TSH percentiles (5th, triangles; 50th, circles; and 97.5th, squares) for the eight age groups for each ethnicity: left, B; middle, W; right, M. Solid symbols represent the TSH percentiles for antibody-positive subjects, and open symbols represent the TSH percentiles for antibody-negative subjects. In each panel, the solid line links the prevalence of TPOAb and/or TgAb of each age group.
Disorders of the Thyroid Gland
The United States Preventive Services Task Force concluded that there was insufficient evidence to recommend routine screening for thyroid disease in adults. Furthermore, there is no consensus regarding screening in the elderly. A scientific review panel suggests case finding for screening older patients at high risk (eg, family history, personal history, radiation, and other risk factors). The American Thyroid Association recommends that adults be screened by serum TSH determination every 5 years. A consensus statement from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society recommends routine screening for subclinical thyroid dysfunction in adults. However, it is reasonable to measure TSH at any time in older individuals who present with atypical symptoms of thyroid disease such as exacerbation of cardiac symptoms, change in mental status, falling, or onset of depression. Despite the sensitivity of the TSH assay, further evaluation with a free T4 or total T4 is often required because up to 98% of elderly subjects with mildly suppressed TSH levels do not have thyrotoxicosis.
With age, the prevalence of Graves disease decreases (although it remains the most common cause of hyperthyroidism), and the prevalence of multinodular goiter and toxic nodules increases. Elderly hyperthyroid patients tend to present with symptoms or complications related to the most vulnerable organ system—usually the cardiovascular system (atrial fibrillation, congestive heart failure, angina, and acute myocardial infarction) or the central nervous system (apathy, depression, confusion, or lassitude). The hypercatabolic state also causes muscle wasting, particularly of the quadriceps, thereby increasing the risk of falls. Occasionally, hyperthyroid elderly patients present with gastrointestinal symptoms, but these differ from those seen in younger patients because they include constipation, failure to thrive, anorexia, and weight loss. Because of degeneration of the sinus node and fibrotic changes in the cardiac conduction system, older patients are less likely than younger ones to present with palpitations (Table 23–1). However, a very low serum TSH concentration is associated with a threefold higher risk of atrial fibrillation in the subsequent decade. Hyperthyroidism is also associated with bone loss and fractures.
| Old1 | Old2 | Young | |
|---|---|---|---|
| Number | 25 | 85 | 247 |
| Mean age | 81.5 | 68.6 | 40a |
| Range | 75-95 | 60-82 | 5-73 |
Symptoms (%) | |||
Weight loss | 44 | 35 | 85 |
| Palpitations | 36 | 42 | 89 |
| Weakness | 32 | 28 | 70 |
| Dizziness, syncope | 20 | … | … |
| Nervousness | 20 | 38 | 99 |
| No symptoms | 8 | … | … |
| Memory loss | 8 | … | … |
| Tremor | 8 | … | … |
| Local symptomsb | 8 | 11 | … |
| Pruritus | 4 | 4 | … |
| Heat intolerance | 4 | 63 | 89 |
Physical Findings | |||
Pulse >100/min | 28c | 58 | 100 |
| Atrial fibrillation | 32 | 39 | 10 |
| New-onset atrial fibrillation | 20d | … | … |
| Lid lag | 12 | 35 | 71 |
| Exophthalmos | 8 | 8 | … |
| Fine skin | 40 | 81 | 97 |
| Tremor | 36 | 89 | 97 |
| Myopathy | 8 | 39 | … |
| Hyperactive reflexes | 24 | 26 | … |
| Gynecomastia | (1 male) | 1 | 10 |
| None | 8 | … | … |
Thyroid Examination (%) | |||
Impalpable or normal | 68 | 37 | … |
| Diffusely enlarged | 12 | 22 | 100 |
| Multinodular goiter | 12 | 20 | … |
| Isolated nodule | 8 | 21 | … |
The physical signs of hyperthyroidism also differ in the elderly (see Table 23–1). Sinus tachycardia is less frequent; the thyroid feels normal in size or is not palpable in two-thirds of patients; and lid lag is uncommon. Ophthalmopathy is less common, not only because Graves disease occurs less often, but also because, even with Graves disease, ophthalmopathy occurs less frequently in the elderly. However, although they are less common in the elderly, some findings appear to be highly suggestive of hyperthyroidism. These include increased frequency of bowel movements, weight loss despite increased appetite, fine finger tremor, eyelid retraction, and increased perspiration. Lid lag is uncommon but if found, it is highly suggestive of hyperthyroidism (specific).
As in younger patients (Chapter 7), the diagnosis is usually confirmed by standard thyroid function tests, beginning with a depressed TSH level as measured by a sensitive immunoradiometric or chemiluminescent assay. There are potential pitfalls, however. Hospitalized elderly patients who are acutely ill (but euthyroid) may have a suppressed serum TSH. Further, other thyroid function tests should help rule out hyperthyroidism. T3 toxicosis may be more difficult evaluation to diagnose because concomitant nonthyroidal illness is common and can depress serum T3.
Furthermore, iodide-induced hyperthyroidism, reflecting the Jod-Basedow phenomenon (see Chapter 7), is becoming more common in elderly patients with multinodular goiter because of increased exposure to radiocontrast studies; the resultant hyperthyroidism is generally transient.
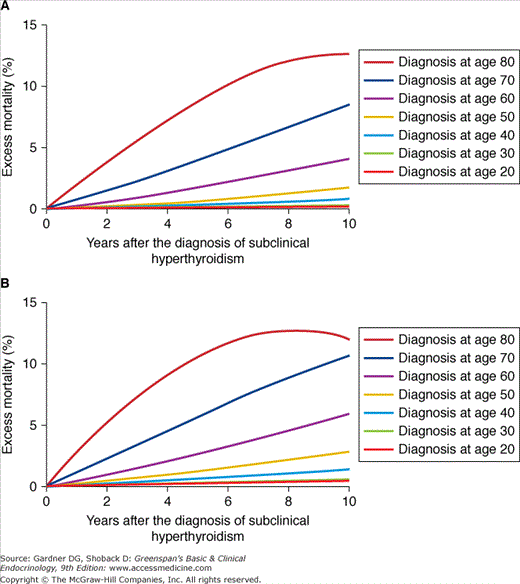
Finally, because of screening tests with sensitive TSH assays, subclinical hyperthyroidism (normal T4, T3, free T4 with a suppressed TSH) is being recognized more commonly. This is often found in older subjects with autonomous function of a multinodular goiter or nodule. Osteoporosis and atrial fibrillation are complications of subclinical hyperthyroidism that may be an indication for treatment. Recent studies suggest a possible increase in all-cause mortality in patients with subclinical hyperthyroidism with an increase beyond the age of 60, especially in aging men (Figure 23–5). If patients are not treated, careful follow-up is recommended.
Figure 23–5
A: Excess mortality from all causes after the diagnosis of subclinical hyperthyroidism for US women and B: US men with subclinical hyperthyroidism. These data are based on a hypothetical approach using mathematical modeling, that is, these data are not based on individual patient data, but derive from aggregated data reported in original cohort studies and life tables.
Beta-blocking agents are useful in alleviating symptoms, but radioactive iodine is the therapy of choice in elderly patients because it is efficient, uncomplicated, and inexpensive. Antithyroid drugs can be used prior to radioactive iodine treatment to render the patient euthyroid and to avoid radiation-induced thyroiditis, but they are not definitive treatment and are more toxic in this age group. Surgery has a more limited role because of its increased morbidity.
Following radioactive iodine treatment, patients become euthyroid over a period of 6 to 12 weeks. They should receive careful follow-up, because hypothyroidism develops in 80% or more of patients who have been adequately treated. Once hyperthyroidism has abated, the metabolic clearance rate of other medications may decrease, and doses may require readjustment. Older patients with subclinical hyperthyroidism require follow-up, especially if presented with an iodine load.
Hypothyroidism in the elderly is most often due to Hashimoto thyroiditis, prior thyroid surgery, or radioactive iodine ablative therapy. Medications, including amiodarone, lithium, and interferon-α, can lead to hypothyroidism. The risk of developing hypothyroidism is significantly increased in older women when serum antithyroid antibodies or serum TSH are elevated.
It is easy to overlook hypothyroidism in an older person, because many euthyroid elderly patients have the same symptoms. Moreover, elderly patients with hypothyroidism are more likely than younger patients to present with cardiovascular symptoms (eg, congestive heart failure or angina) or neurologic findings (eg, cognitive impairment, confusion, depression, paresthesias, deafness, psychosis, or coma). Finally, in the older hypothyroid patient, the physical findings are frequently nonspecific, although puffy face, delayed deep tendon reflexes, and myoedema support the diagnosis.
Serum TSH is the most sensitive indicator of primary hypothyroidism and should be checked first. The diagnosis should then be confirmed with a low serum T4 or free T4. Measurement of serum T3 is unnecessary and potentially misleading, because T3 is the thyroid hormone most likely to decrease in nonthyroidal illness. Serum TSH should not be used alone to diagnose hypothyroidism because it does not always differentiate symptomatic from subclinical hypothyroidism. Furthermore, levels of TSH may be higher at night as a result of the nocturnal rise in serum TSH. Moreover, the pulsatile nature of TSH, resulting in serum TSH slightly above the normal ranges, may lead to a diagnosis of subclinical hypothyroidism in a euthyroid subject. Finally, in hypothyroid patients, serum TSH levels can be reduced to within the normal range by treatment with dopaminergic drugs and corticosteroids. In such patients, determination of free T4 may help differentiate those with true hypothyroidism from those with nonthyroidal illness (Chapter 7).
The doses of thyroid hormone required for adequate replacement decrease with age. Elderly patients should be started on approximately 25 to 50 μg of levothyroxine, and the dose should be increased by approximately 25 μg every 4 to 6 weeks until the serum TSH comes into the normal range. In patients with cardiovascular disease, even lower initial doses can be used (12.5 μg) and increased at a slower rate. Desiccated thyroid hormone and preparations containing T3 should be avoided, because T3 is rapidly absorbed and cleared and may be cardiotoxic. The metabolic clearance of other drugs changes as hypothyroidism is corrected, and their dosages may require readjustment. On average, the dose of levothyroxine in the elderly is roughly 1 μg/kg/d compared with 1.7 μg/kg/d in young adults. Overtreatment documented by a suppressed serum TSH should be avoided because of the potential adverse effects to the skeleton and cardiovascular system.
It is still not known whether treating subclinical hypothyroidism is beneficial. A Cochrane review of 12 small trials that included treatment of subclinical hypothyroidism with thyroid hormone did not find improved survival, decreased cardiac morbidity, or differences in quality of life or symptoms. Two-thirds of patients with subclinical hypothyroidism were chemically euthyroid for at least 4 years, and the absence of antimicrosomal antibodies may identify patients at lowest risk of progression. The presence of antithyroperoxidase antibodies is associated with a higher risk of progression to overt hypothyroidism of 4.3%/year versus 2.6%/year in antibody-negative patients.
If patients are not treated, they need to be followed closely for progression of disease. Elderly patients previously treated with radioactive iodine are more likely to progress to overt hypothyroidism.
The prevalence of multinodular goiter increases with age. However, if swallowing and breathing are not compromised and thyroid function tests are normal, the goiter can be observed without treatment. Levothyroxine therapy rarely shrinks the gland, and although it may prevent further enlargement, the risk of inducing iatrogenic hyperthyroidism is significant. This is compounded by the fact that multinodular goiters may independently develop areas of autonomous function.
Thyroid nodules are more common in the elderly. The prevalence of nodules increases with age and is generally higher in women than in men. Furthermore, the prevalence of thyroid nodules detected by ultrasound may be as high as 40% in older women. Ninety percent of these nodules are benign, but the prognosis for elderly patients with malignant nodules may be worse than that for younger patients with malignant nodules. The approach is similar to the workup in a younger patient. The prognosis correlates with the size of the tumor. The outcome in elderly patients may therefore be substantially improved by early evaluation of nodules in patients who are good surgical candidates.
Papillary carcinoma is more common in young and middle-aged patients. However, it has a poorer prognosis in the elderly, possibly because it is detected at a more advanced stage. Follicular carcinoma accounts for 15% of thyroid cancers and usually occurs in middle-aged and older patients. Anaplastic thyroid carcinoma is found almost exclusively in middle-aged and older patients. It presents as a rapidly growing hard mass that is locally invasive, often associated with metastatic lesions, and has a very poor prognosis (Chapter 7).
Carbohydrate Intolerance and Diabetes Mellitus
Diabetes is an important health condition for the aging population. More than 20% of individuals over the age of 60 years have diabetes, and another 14% have impaired glucose tolerance (IGT). It is anticipated that these numbers will continue to increase in the coming decades. It is therefore almost certain that those involved in the care of patients in this age group will encounter those with some disorder of glucose tolerance.
Both IGT and diabetes are associated with enhanced morbidity and mortality due to macrovascular and cardiovascular disease (CVD); an increase in risk for microvascular complications (retinopathy, neuropathy) that impair the ability to maintain independence; changes in cognitive function; and depression. Older individuals with diabetes have more functional disability and coexisting illnesses such as hypertension, coronary heart disease (CHD), and stroke than those without diabetes. Older adults with diabetes are also at greater risk for urinary incontinence, injurious falls, and issues related to polypharmacy.
Early diagnosis and appropriate intervention can help to avoid some of the adverse consequences of hyperglycemia, while also improving health-related quality of life (HRQL) in this age group. In 2003, the American Geriatric Society published guidelines that are useful in guiding the care of the older person with diabetes. These recommendations are now included as part of the standards of care published annually by the American Diabetes Association.
Normal aging is associated with a gradual increase in both fasting (1 mg/dL [0.6 mmol/L] per decade) and glucose-stimulated blood glucose (BG) (5 mg/dL [0.28 mmol/L] per decade) levels, even in lean, physically active individuals with normal body weight. Normoglycemic elderly subjects have higher glucose and insulin levels during an oral glucose tolerance test (OGTT) than body mass index (BMI)-matched young subjects. These changes in BG are more profound in those who have risk factors for abnormal glucose tolerance.
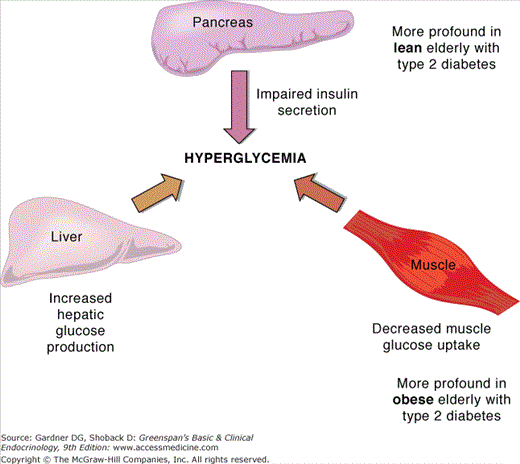
Type 2 diabetes is characterized by defects in both insulin secretion and sensitivity. Although the majority of people with type 2 diabetes are insulin-resistant, insulin resistance by itself is not sufficient to lead to hyperglycemia. Age-related impairments in beta cell function that include reduced conversion of proinsulin to insulin and abnormal insulin secretion are important contributors to the development of hyperglycemia in elderly individuals. Lean elderly patients with type 2 diabetes have a greater impairment in insulin secretion than insulin sensitivity while obese patients have both impaired insulin secretion and sensitivity in muscle and liver (Figure 23–6).
Several abnormalities of beta cell function contribute to the decline in insulin secretory capacity with advancing age. These include an accumulation of intracellular lipid within the beta cell; a decrease in glucose transporter (GLUT) 2 number; and a reduced sensitivity to the incretin hormones, glucagon-like peptide 1 (GLP1), and glucose insulinotropic peptide (GIP) that acts to augment the postprandial insulin response. Genetic susceptibility also plays a role. In individuals without diabetes who carry five or more risk alleles that are associated with development of diabetes, impairments in insulin secretion are observed at an earlier age than those with fewer alleles. Evidence of autoimmunity with antibodies directed against beta cell proteins has been demonstrated as a cause of impaired insulin secretion in up to 12% of elderly individuals.
These impairments in beta cell function can be unmasked by an increase in insulin resistance that occurs with normal aging. The insulin resistance that accompanies aging is associated with the accumulation of intracellular fat and a progressive decline in the number and function of mitochondria resulting in reduced oxidative glucose metabolism and phosphorylation activity. Insulin resistance is augmented by weight gain; reductions in lean body mass and increases in fat mass; physical inactivity; and use of some medications (ie, glucocorticoids and atypical antipsychotics). Insulin resistance occurs in muscle, where there is a reduction in insulin-mediated glucose uptake following a meal; in adipose tissue where impaired regulation of hormone-sensitive lipase results in increases in circulating levels of free fatty acids (FFA); and liver where there is overproduction of glucose in the fasting state and failure to adequately suppress glucose production after a meal.
The metabolic syndrome refers to a clustering of metabolic abnormalities outlined in Table 23–2 that are associated with an increase in risk for type 2 diabetes and CVD. This syndrome is more common in the elderly, increasing from approximately 7% of the population in the third decade to 44% in the seventh decade. Lifestyle interventions targeting modifications in dietary intake, regular exercise, and weight reduction are effective in reducing the progression to overt type 2 diabetes among individuals above age 60 years.
Diagnosis requires three of the following:
|
The majority of elderly individuals with diabetes will have type 2 diabetes. The typical symptoms usually associated with hyperglycemia may either not be present or may have developed so gradually that they may not be perceived as being abnormal. The use of diuretics can mask polyuria and polydipsia as manifestations of hyperglycemia. An age–related decrease in thirst may blunt these usual symptoms. It is therefore important that laboratory measurement of BG be performed on at least an annual basis in individuals above the age of 60.
A diagnosis of diabetes can be established by one of three established criteria outlined in Table 23–3. Oral glucose tolerance testing is performed infrequently in clinical practice and is not required to establish the diagnosis, particularly in elderly individuals. Measurement of an A1c is now accepted as an alternative tool in establishing a diagnosis of diabetes, with values ≥6.5% consistent with a diagnosis of diabetes. A1c values of 6.0% to 6.4% identify those at high risk for development of type 2 diabetes. A reasonable approach would be to measure an A1c in any elderly person with abnormal glucose values.
Both the ADA and the American Geriatrics Society recommend that functional, cognitively intact older adults who have significant anticipated life expectancy be treated to similar glycemic goals as younger adults. This means guiding therapy to achieve HbA1c of <7% for the majority of people with diabetes. Achieving this level requires that fasting glucose levels be maintained between 80 and 130 mg/dL, and 2-hour postprandial BG at <180 mg/dL. These recommendations are not absolute. While the goal is to maintain an A1c values as close to normal (<6%) as possible, this goal can be modified in patients who are at high risk for hypoglycemia, who are older, who have comorbid conditions that limit life expectancies. These contingencies have particular relevance to the elderly population who may experience variability in appetite or food intake, or often have other medical problems requiring medications (eg, beta blockers, anxiolytics) that can increase vulnerability to hypoglycemia. On the other hand, uncontrolled diabetes is also associated with undesirable effects in elderly individuals, including fluid and electrolyte abnormalities, problems with urinary incontinence and cognitive function, indicating the need for a reasonable degree of glycemic control even with advanced age. Efforts to achieve A1c levels of <7% are reasonable in some elderly patients, provided that the risk for weight gain and hypoglycemia is minimized. Therapy can be modified to achieve A1c targets of 8% in others as a way of minimizing risk for treatment-associated concerns. However, symptomatic hyperglycemia that can increase risk for an acute hyperglycemic crisis should be avoided in all patients.
Because the complications of diabetes mellitus are related to the duration of disease, elderly patients who live long enough suffer the same complications of nephropathy, neuropathy, and retinopathy as their younger counterparts. The United Kingdom Prospective Diabetes Study (UKPDS) examined the relationship between improved glycemic control and the prevention of complications in 3067 patients with type 2 diabetes (mean age 54 years) assigned to intensive (goal of fasting blood glucose <108 mg/dL) or conventional therapy. After a median follow-up of 10 years, the incidence of microvascular complications (retinopathy, neuropathy, and nephropathy) was reduced by 25% with intensive treatment. There was no difference in the incidence of macrovascular complications, but there was a tendency for fewer myocardial infarctions in the intensive therapy group. A 10-year follow-up report from the UKPDS demonstrated a significant favorable legacy effect of early intensive therapy in preventing both microvascular and macrovascular complications.
Elderly diabetic patients have an impaired counterregulatory response to hypoglycemia which can interfere with recovery from a hypoglycemic event. In addition, the ability to sense hypoglycemia declines, as does the ability to take corrective action. Coupled with the diminished cortical reserve due to the higher prevalence of age-associated conditions such as stroke, lacunar infarction, amyloid angiopathy, and Alzheimer disease, the older brain is less able to recover fully from hypoglycemic insult.
CVD is a major cause of morbidity and mortality in elderly people with diabetes. For this reason, interventions recommended for the secondary prevention of recurrent coronary events in individuals without diabetes are recommended for all individuals with diabetes independent of known heart disease. This includes control of blood pressure, treatment of hyperlipidemia, and use of low-dose aspirin therapy in those without contraindications. While tight glycemic control has been demonstrated to reduce the risk for microvascular and neuropathic complications, the role of tight glycemic control for prevention of macrovascular disease and CVD has been addressed in several recent clinical trials.
Three recent large, randomized, prospective clinical trials with relevance to an elderly population were conducted in an effort to define the effect of lowering A1c to levels lower than those currently recommended by the ADA on cardiovascular outcomes in patients with type 2 diabetes. The average age of these subjects, all of whom had a history of a CVD event or were at high risk for an event, ranged from 60 to 66 years.
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, the goal of intensive glycemic therapy was to achieve an A1c <6% versus <8% with conventional treatment. The glycemic control study was stopped 18 months early due to the observation of a 22% increase in the relative risk for CVD death with intensive therapy. In the Action in Diabetes and Vascular Disease (ADVANCE) study, the goal of intensive therapy was to achieve an A1c <6.5% using the sulfonylurea, gliclazide (not available in the United States). A reduction in microvascular complications, primarily risk for proteinuria, was observed with intensive therapy, but there were no group differences for CVD events. In the Veterans Affairs Diabetes Trial (VADT), the goal of intensive therapy was to achieve an A1c <6%. There were no significant group differences in the composite outcome of CVD events; however, there were more CVD deaths (36 vs 29) and more sudden deaths in intensively treated subjects (11 vs 4) (p = NS). Severe hypoglycemia within the preceding 90 days was a predictor of CVD events and mortality.
What do these results mean to the practicing physician? It is important to note that all three trials included participants with long-standing type 2 diabetes and varying degrees of established atherosclerosis at time of study entry. In addition, the glycemic targets in these trials were well below what is recommended by the ADA (7%) and may not be appropriate in elderly individuals where the risk for hypoglycemia and related adverse sequellae is greater. Other CVD risk factors (eg, hypertension and hyperlipidemia) were treated to a moderate or high degree, resulting in rates of CVD events that were lower than predicted with standard therapy. This places emphasis on the importance of treating nonglycemic CVD risk factors in patients with diabetes.
The therapeutic goals for any patient with diabetes, including the elderly, are to avoid symptoms of hyperglycemia and hypoglycemia, to minimize risk for acute and chronic diabetes-related complications, and to achieve an optimal health-related quality of life. While the majority of newly diagnosed elderly individuals will have type 2 diabetes, many individuals with type 1 diabetes are enjoying longer survival, making it likely that these patients will require care during advancing years. Insulin therapy, either alone or in combination with oral agents, is often required to maintain desired glycemic control in the face of progressive beta cell dysfunction in those with type 2 diabetes. For these reasons, knowledge of insulin and noninsulin injectable medications is as important as that of the five different classes or oral hypoglycemic agents (Table 23–4) indicated for the treatment of type 2 diabetes.
| Class | Mechanism Action | Side Effects | Agents |
|---|---|---|---|
| Sulfonylureas | Stimulates insulin production | Hypoglycemia |