In the United States, one in eight women (13%) will develop invasive breast cancer during her lifetime. In 2021, it was estimated that 281,550 new cases of invasive breast cancer would be diagnosed in women in the United States, and 49,250 women would be diagnosed with noninvasive or in situ disease. It was expected that 43,600 women in the United States (about twice the seating capacity of the Madison Square Garden arena in New York City) would die from breast cancer in 2021. While mortality from breast cancer in women under the age of 50 has remained steady since 2007, mortality from breast cancer in women over the age of 50 has continued to decrease by a rate of 1% per year between 2013 and 2018. It is felt that this reduction in breast cancer mortality is related to earlier detection due to increased screening efforts, as well as advances in therapy. Given that prognosis after breast cancer is dependent on stage, early detection or, even better, prevention can make an enormous impact for an individual. This is where risk assessment and management can make a tremendous difference.
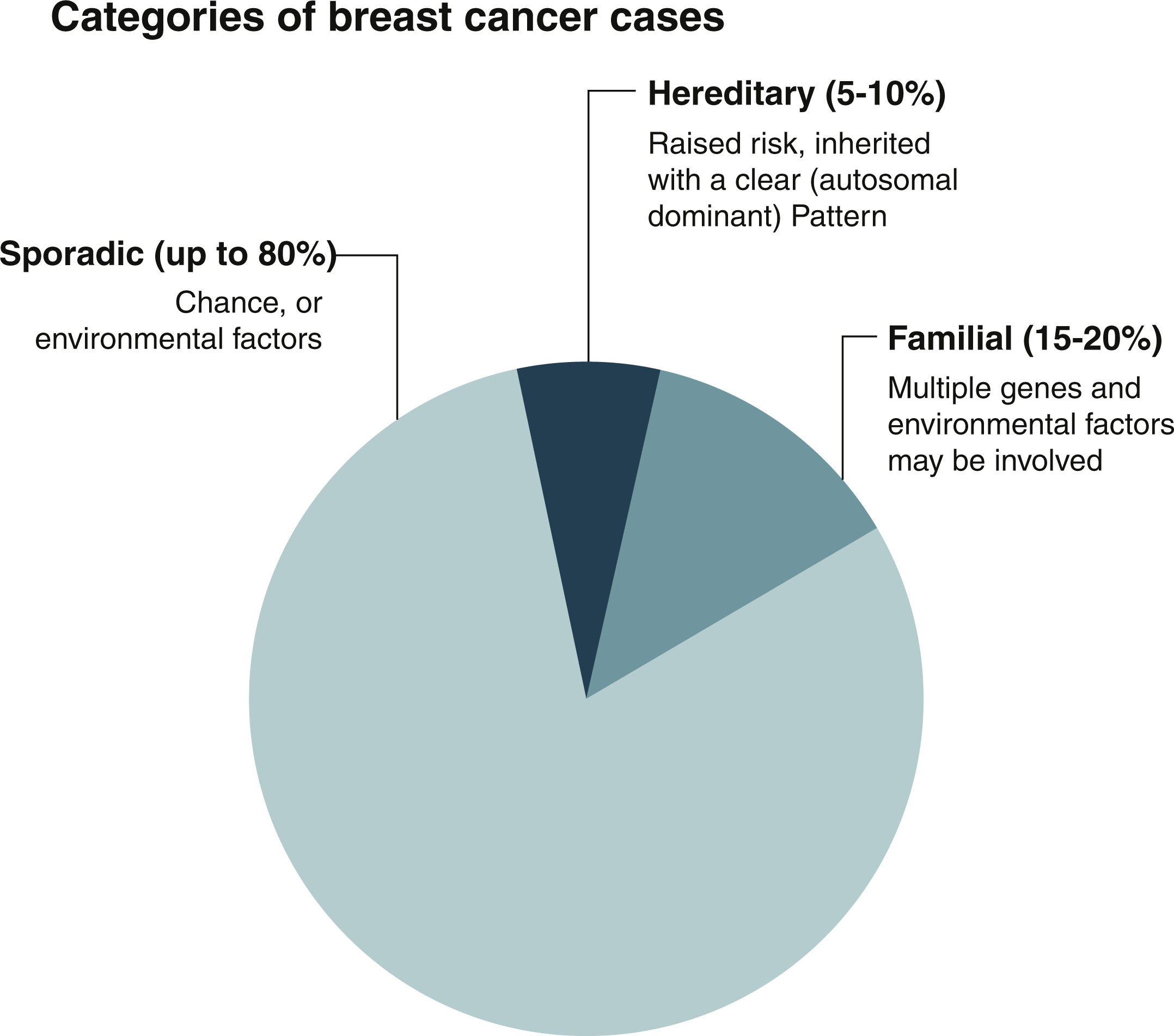
Approximately 80% of breast cancer is considered to be sporadic and occurs in women who have little to no family history of cancer. These cancers are thought to develop secondary to acquired genetic pathogenic variants that occur because of the aging process and environmental factors. The biggest risks for breast cancer across the population are age and female sex. Familial breast cancer accounts for 15% to 20% of all breast cancer, and it is thought to be caused by multiple genes and environmental factors combined. Only about 5% to 10% of breast cancer is secondary to an inherited germline pathogenic variant ( Fig. 15.1 ). There are opportunities for lifestyle modification that can decrease a woman’s risk of breast cancer and should be considered for even an average-risk individual. However, there is a contingent of patients who are at elevated risk for breast cancer due to personal and family history, and who would benefit from increased screening, risk-reducing medications, risk-reducing surgery, and lifestyle modifications. The goal of this chapter is to help the clinician identify and manage the patient population that is at high risk for developing breast cancer, which is defined as a lifetime risk for breast cancer greater than 20% or a 5-year risk of breast cancer greater than 1.67%. The first step in identifying a patient that is at high risk for breast cancer is looking at the individual’s genetic risk factors, personal and family risk factors, and lifestyle risk factors.
Genetic Risk Factors
The National Cancer Care Network (NCCN) guidelines for genetic assessment in patients without a history of breast cancer recommends consideration for genetic counseling and possible testing for patients with a close relative, meaning a first- or second-degree relative, with one of the following: breast cancer diagnosed before the age of 45, two breast cancer primaries in a single individual, two individuals with breast cancer primaries on the same side of the family with one of those individuals being diagnosed before the age of 50, ovarian cancer, male breast cancer, pancreatic cancer, or metastatic prostate cancer. Individuals should also be considered for genetic counseling and testing if they have a personal or family history of three or more of the following cancers: breast cancer, sarcoma, adrenal corticoid carcinoma, brain tumor, leukemia, colon, endometrial, thyroid, or kidney cancer, macrocephaly, hamartomatous gastrointestinal (GI) polyps, lobular breast cancer, diffuse gastric cancer, gastrointestinal cancer, pancreatic cancer, ovarian sex cord of testicular Sertoli cell tumors, or childhood skin pigmentation. Individuals that meet the above criteria should be referred for formal genetic assessment and counseling.
Most hereditary breast cancer is caused by pathogenic variants in the BRCA1 and BRCA2 genes (BReast CAncer gene 1 and BReast CAncer gene 2). These genes are an important component in a deoxyribonucleic acid (DNA) repair pathway called homologous recombination (HR). HR is a process in which a strand of DNA is repaired using the homologous strand as a template. Loss of function of one of the copies of either of the BRCA genes leads to a defect in HR. The resulting inability of the cell to repair the DNA leaves it open to further damage, thus increasing the risk for cancer.
Over a woman’s lifetime, the cumulative risk for breast cancer in a BRCA1 pathogenic variant carrier is 55% to 72%. BRCA2 pathogenic variant carriers have a lifetime risk of 45% to 69%. The range in risk has not yet been fully explained. Currently, polygenic risk scores are in development attempting to determine if otherwise benign variants in other genes modify the risk of pathogenic variants in the BRCA genes. It is likely that other nongenetic factors also influence this risk.
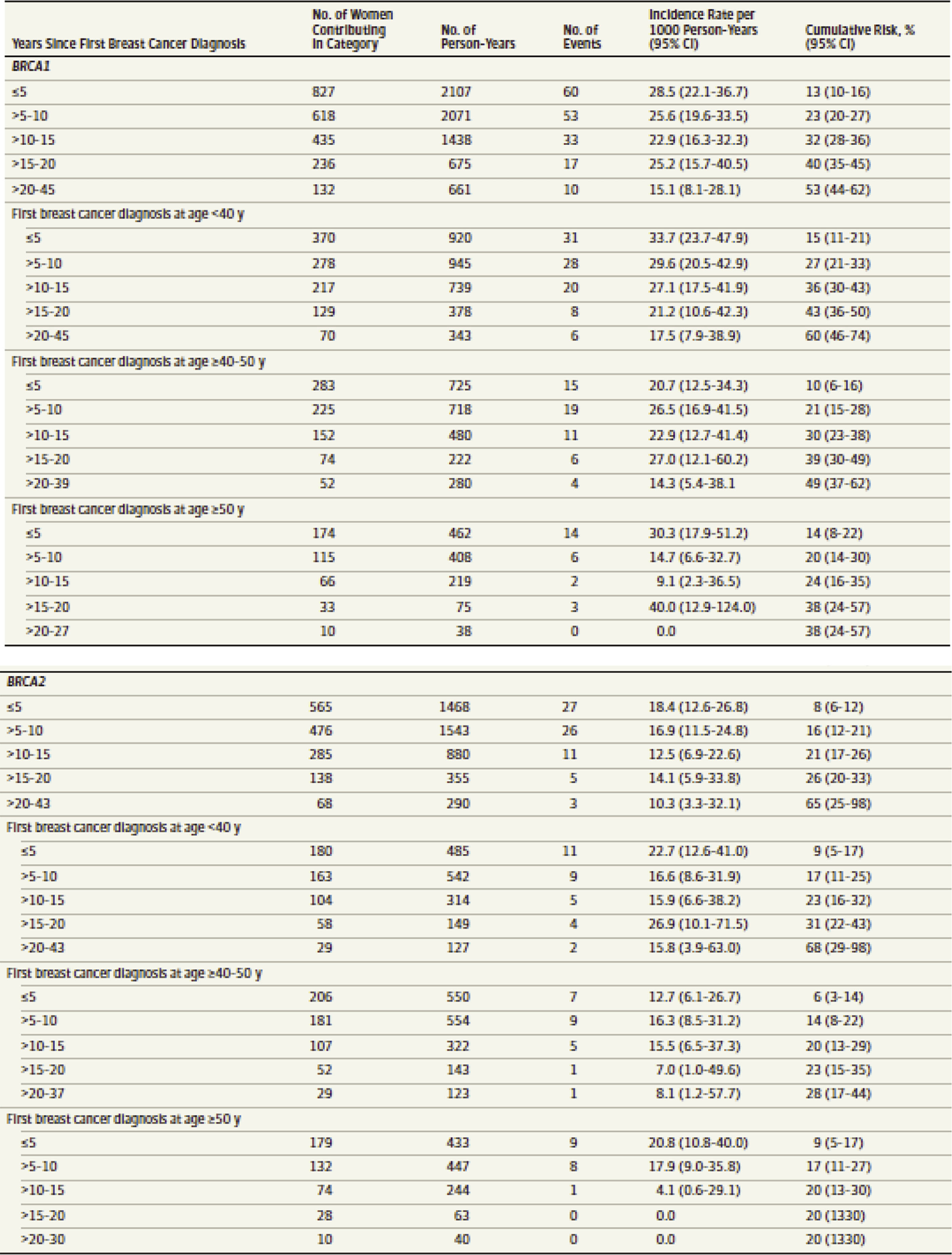
Women with pathogenic variants in one of the BRCA genes also have an increased risk of developing a second primary breast cancer. This risk is dependent upon the age at which the woman was when her first breast cancer was diagnosed, as well as which BRCA gene is altered. The risk for a second primary breast cancer is thought to be 21.1% within 10 years and up to 83% by age 70 in BRCA1 carriers. For BRCA2 carriers, 10.8% within 10 years and 62% by age 70. Still, the ability to further stratify breast cancer risk by age for BRCA pathogenic variant carriers can be useful in surgical decision making. Patients can use more narrow risk assessments in determining whether they are comfortable with breast-conserving therapy versus bilateral mastectomy. Tables such as the one in Fig. 15.2 can be used as counseling aids in this decision-making process.
It is important to note that pathogenic variants in the BRCA genes also increase the risk for other cancers by the same HR deficiency mechanism. For women, the risk for ovarian cancer is between 39% and 44% for BRCA1 carriers and 11% to 17% for BRCA2 carriers. Additionally, there are increased risks for fallopian tube cancer, primary peritoneal cancer, and pancreatic cancers. Men have an increased risk for prostate cancer.
Although the BRCA genes cause the majority of hereditary breast cancer, there are other genes in which pathogenic variants can cause varying degrees of breast cancer risk. These genes are often categorized by their lifetime risk for breast or other cancers, also known as penetrance. The genes with the highest penetrance in addition to the BRCA genes are tumor protein p53 (TP53), Cadherin 1 (CDH1), Partner and Localizer of BRCA1 (PALB2), phosphatase and tensin homolog deleted (PTEN), and serine/threonine kinase 11 (STK11). Pathogenic variants in these genes are associated with lifetime risks for breast cancer that can reach 50% or higher. The moderate penetrance genes have been more recently described, and our understanding of them is likely incomplete. Additionally, management recommendations vary for the moderate penetrance genes. For example, ataxia telangiectasia mutated (ATM), BRCA1 Associated Ring Domain 1 (BARD1), Checkpoint Kinase 2 (CHEK2), and Neurofibromatosis type 1 (NF1) all have an absolute risk for breast cancer of 15% to 40%, and the NCCN makes recommendations for high-risk screening for breast cancer in individuals with pathogenic variants in these genes. Still, other moderate penetrance genes such as Restriction Site Associated DNA Paralog C and Paralog D (RAD51C and RAD51D) also have a lifetime risk for breast cancer in the range of 15% to 40% ; however, the NCCN recommends that screening for breast cancer be based on other factors such as family history.
Genetic testing for genes related to increased risks for breast and other cancers is most commonly done via next-generation sequencing (NGS). This technology allows for the testing of multiple genes simultaneously, which renders results in a more timely manner than testing genes individually and sequentially. Still, testing multiple genes can result in complex results. Some patients may be found to have pathogenic variants in more than one gene. Patients can have variants within genes that are of unknown clinical significance. Even patients with single pathogenic variants in genes will need care beyond breast cancer risk management. It is imperative that patients understand the potential complexities of genetic testing. Because of this, the National Program of Breast Centers, the body that sets the standards and accredits breast cancer treatment centers in community care, has set a standard for genetic counseling and testing in breast cancer. Standard 2.16 says that pretest genetic counseling should be performed by a cancer genetics professional who has an educational background in genetics and cancer genetics, counseling, and hereditary cancer syndromes and can provide accurate risk assessment and empathetic genetic counseling to cancer patients and their families. The National Society of Genetic Counselors has a resource to find qualified genetic counselors who can provide this evaluation both in person and virtually ( https://findageneticcounselor.nsgc.org/ ).
Familial/Personal History/Lifestyle Risk Factors
If an individual does not meet the above criteria for genetic counseling and testing, they should then be assessed for breast cancer risk factors related to personal and family history. There are several different computer models that can be used to calculate a woman’s risk of breast cancer. One of the earliest computer models to look at breast cancer risk is the modified Gail model. This model is available at the National Cancer Institute website at http://www.cancer.gov/bcrisktool/Default.aspx . The modified Gail model is based on patient age, age at menarche, age of first live birth, number of biopsies, presence of atypia (but not lobular carcinoma in-situ [LCIS]), and the number of first-degree relatives with breast cancer. The modified Gail model is only validated to use in patients age 35 or older. The NCCN Risk Reduction Panel recommends that those individuals greater than age 35 with a 1.66% or greater 5-year risk of breast cancer be considered for risk reduction strategies such as lifestyle modification and risk-reducing medication. The Gail model is not without limitations. It should not be used in patients with a BRCA1 , BRCA2 , PTEN , or P53 mutation. It is not recommended for individuals with a strong family history of breast cancer, a history of thoracic radiation between the ages of 10 and 30, or those individuals with lobular carcinoma in situ.
The Tyrer-Cuzick model is a computer-based multivariant model that considers personal history, history of atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), and classical LCIS, family history of both the maternal and paternal sides and breast density. The Tyrer-Cuzick model can be found online at http://www.ems-trials.org/riskevaluator/ . Individuals with a lifetime risk for breast cancer of greater than 20% based on the results of the Tyrer-Cuzick calculation can be offered increased screening with breast magnetic resonance imaging (MRI), risk-reducing medication, and recommendations regarding lifestyle modification. Unlike the Gail model, the Tyrer-Cuzick risk model can be used in individuals less than 35 years of age. An analysis of the Mayo Clinic cohort demonstrated that the Gail model underestimated the risk of breast cancer in individuals with atypical hyperplasia, while the Tyrer-Cuzick risk model overestimated the risk of breast cancer in individuals with a personal history of ALH/ADH.
Other elements that can cause an increased breast cancer risk include being born female, increasing age, ethnicity, and race, and lifestyle factors including increased body mass index (BMI), alcohol consumption, current or prior estrogen-progesterone hormone therapy, and reproductive history. The two greatest risk factors for developing breast cancer are being born female and increasing age. While men can develop breast cancer, the incidence is much higher in women, and most breast cancers are diagnosed in women aged 55 and older. Ethnicity and race also play a factor in breast cancer risk. Caucasian women have a slightly higher risk of breast cancer than African American women. African American women have a higher incidence of breast cancer being diagnosed before the age of 45 and are more likely to die from breast cancer at any age. Asian, Native American, and Hispanic women have a lower incidence and mortality rate from breast cancer. While age, race/ethnicity, and family history are beyond the control of the individual, clinicians can educate and affect many of the breast cancer risk factors related to lifestyle.
The prevalence of obesity among adults in the United States in 2017 to 2018 was 42.4%. Among women, the prevalence of obesity was 39.7% among those aged 20 to 39, 43.3% among those aged 40 to 59, and 43.3% among those aged 60 and over. There is a substantial amount of evidence that overweight/obese women have a higher risk for postmenopausal breast cancer. The Nurses’ Health Study suggested that women experiencing a weight gain of 25 kg or more since the age of 18 have an increased risk of breast cancer compared to those who have maintained their weight. Most breast cancer diagnosed in post-menopausal individuals is hormone sensitive, specifically estrogen receptor and/or progesterone receptor sensitive. After menopause, once an individual’s ovaries have stopped producing estrogen, most of the estrogen production comes from the fat tissue. Having more fat tissue increases estrogen levels and increases the risk of hormone-positive breast cancer. In the same Nurses’ Health Study, women who never used postmenopausal hormone replacement therapy and lost 10 kg or more after menopause and were able to keep the weight off had a significantly lower risk for breast cancer compared to those who maintain that weight.
Exercise has been associated with decreased risk of breast cancer. A population, case-controlled study of 4538 patients with breast cancer and controlled patients grouped according to race demonstrated a 20% lower risk of breast cancer in both black and white women with annual lifetime activity levels greater than the median activity level when compared to inactive women. The American Cancer Society recommends that adults get between 150 and 300 minutes (about 5 hours) of moderate-intensity or 75 to 150 minutes of vigorous-intensity exercise per week, with reaching 300 minutes or more of exercise per week being the ideal.
There is a known correlation between alcohol intake and breast cancer risk. A population-based study of 51,847 women provided an association between alcohol consumption and an increased likelihood of developing hormone-positive breast cancer. Even one drink per day can increase breast cancer risk. In an analysis of two cohort studies, there was found to be a 10% increase in risk for breast cancer for every 10 g of alcohol consumed daily. The consensus from the NCCN risk reduction panel was to recommend less than one alcoholic beverage per day. One drink is defined as 1 oz of liquor, 6 oz of wine, or 12 oz of beer.
Hormone replacement therapy can also increase an individual’s risk of breast cancer. The Women’s Health Initiative enrolled 161,809 women between the ages of 50 and 79 into a set of clinical trials. Two of these trials involved the use of hormone therapy in primary disease prevention. The first trial of 16,608 involved women with an intact uterus were given estrogen plus progesterone or a placebo. The second trial involved 10,739 women who had undergone prior hysterectomy and were randomized to estrogen therapy alone versus a placebo. The first trial was closed early after a 26% increased incidence of breast cancer was identified in the hormone therapy treatment group. The use of hormone therapy was associated with a greater incidence of breast cancer as well as higher breast cancer mortality. However, the incidence of breast cancer rapidly declined in patients once they had stopped taking hormone therapy. While the second clinical trial looking at estrogen therapy alone did not demonstrate an increased risk for breast cancer, there was an increased incidence of abnormal mammograms associated with breast density, as well as an increase in the incidence of benign proliferative breast changes. Combined hormone replacement therapy as well as estrogen replacement therapy alone has been demonstrated to increase a postmenopausal individuals’ risk for cardiovascular disease (e.g., heart attack and stroke). Women with elevated risk for breast cancer should not be offered hormone replacement therapy. Other means of controlling menopausal symptoms should be attempted prior to resorting to hormone replacement therapy. However, if avoidance of hormone replacement therapy is not possible, patients should be given the lowest dose of hormone replacement for a limited time. Women who are offered hormone replacement therapy need to be counseled that hormone replacement therapy can increase the risk of breast cancer and can make breast cancer more difficult to detect on a mammogram due to changes in breast density.
An individual’s reproductive history can also affect breast cancer risk. Nulliparity as well as a prolonged interval between menarche and an individual’s first live birth, early onset of menarche, or late onset of menopause have been associated with increased risk of breast cancer. Numerous studies have demonstrated a protective effect from breastfeeding, with the relative risk for breast cancer decreasing by 4.3% for every 12 months of breastfeeding performed.
Risk-Reducing Interventions
Tamoxifen
Tamoxifen is a selective estrogen receptor modulator (SERM) that acts as a competitive inhibitor of estradiol. Tamoxifen exerts an antiestrogenic effect on breast tissue and an estrogenic effect on the skeletal system. Tamoxifen’s beneficial effect in the treatment of hormone-positive breast cancer has been well documented. It has also been found to be effective in breast cancer prevention in the high-risk individual. The National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial demonstrated that patients taking 20 mg of tamoxifen daily for 5 years had a 49% risk reduction in the development of invasive cancer and a 50% risk reduction in the development of noninvasive cancer compared to individuals that took a placebo for 5 years.
Tamoxifen may be considered for individuals 35 years and older with a greater than 1.67% 5-year risk for developing breast cancer, based on the validated risk model, or for a woman with a personal history of LCIS. The efficacy of these medications in individuals less than 35 years of age is unknown. The recommended dose of tamoxifen for risk reduction is 20 mg p.o daily for 5 years. A recent randomized clinical trial looked at tamoxifen 5 mg p.o. daily for 3 years in individuals with a personal history of LCIS, ADH, or hormone-positive ductal carcinoma in situ (DCIS). With a median follow-up of 5 years the lower dose of tamoxifen did demonstrate a 50% reduction in the development of invasive cancer or DCIS. The 5 mg daily dose of tamoxifen did not demonstrate an increased incidence of venous thromboembolism (VTE) or endometrial cancer that has been associated with the 20 mg dose. There was also the less reported incidence of vasomotor symptoms compared with the higher dose. While 20 mg daily of tamoxifen is still considered the standard of care, the lower dose may be an option for individual patients.
Tamoxifen side effects include a 1% increased risk of endometrial cancer, especially in women over the age of 50, as well as an increased incidence of vaginal discharge. Tamoxifen also carries an increased risk of ischemic stroke, especially in older patients. It is also associated with an increased incidence of hot flashes.
Tamoxifen is contraindicated in individuals with a personal history of thrombotic events including deep venous thrombosis, pulmonary embolism, ischemic stroke, and transient ischemic attack. It should not be offered to individuals who will be immobilized for extended periods of time and is contraindicated in pregnancy or breastfeeding. Patients should be counseled that it takes approximately 2 months for tamoxifen to clear the body after ingestion. Tamoxifen is not associated with an increased incidence of ischemic heart disease and can lower cholesterol. Patients taking tamoxifen with an intact uterus need to have a baseline pelvic exam followed by a yearly pelvic exam. Patients that developed abnormal vaginal discharge or bleeding need to be worked up immediately to rule out endometrial cancer; the workup should include a transvaginal ultrasound as a minimum.
Raloxifene
Raloxifene is also a SERM. It has an estrogen agonistic effect on the skeletal system and was the first SERM to be approved for the treatment of postmenopausal osteoporosis. Like tamoxifen, it has been shown to decrease serum cholesterol. The recommended dose of raloxifene for risk reduction is 60 mg p.o. daily. Raloxifene is recommended for postmenopausal individuals age 65 and older with a greater than 1.7% 5-year risk for developing breast cancer, based on the validated risk model, or for a woman with a personal history of LCIS. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial demonstrated a decreased risk of invasive breast cancer with tamoxifen use versus raloxifene (50% vs. 38% reduction). Both were equally effective in reducing long-term risk for noninvasive breast cancer.
Raloxifene is associated with an increased risk of VTE. It seems this risk is highest at the initiation of therapy. However, the risk of VTE with raloxifene was lower than the risk of VTE for patients taking tamoxifen in the STAR trial. Raloxifene is not associated with an increased risk of endometrial cancer or sarcoma.
Raloxifene is contraindicated in premenopausal patients, as well as in individuals with a personal history of thrombotic events including deep venous thrombosis, pulmonary embolism, ischemic stroke, and transient ischemic attack. It should not be offered to individuals who are immobilized for extended periods of time and is contraindicated in patients who are taking cholestyramine or estrogen.
Exemestane/Anastrozole Aromatase Inhibitors
Exemestane and anastrozole are steroidal aromatase inhibitors. They irreversibly block the enzyme aromatase which is necessary for estrogen synthesis. By decreasing the circulating amount of estrogen, they affect estrogen-dependent organs such as bone and breast. While the aromatase inhibitors are not FDA (Food & Drug Administration) approved for risk reduction, the NCCN Breast Cancer Risk Reduction Panel did recommend the use of exemestane and anastrozole for risk reduction in postmenopausal women over the age of 35 with a 1.66% 5-year risk of breast cancer based on the Gail model or with a history of LCIS based on the results of the MAP.3 and the IBIS-II trials. The recommended dosing for exemestane is 25 mg p.o. daily. The dose recommendation for anastrozole is 1 mg p.o. daily.
The aromatase inhibitors are contraindicated in premenopausal patients. Side effects include hot flashes, arthralgias, and decreased bone mineral density. Because of the potential loss of bone mineral density, postmenopausal patients taking an aromatase inhibitor need to be followed with a baseline bone mineral density test followed by a yearly evaluation. Postmenopausal patients with osteoporosis may be better candidates for raloxifene, which can target both breast cancer risk reduction and improve bone mineral density.
Managing Hot Flashes or Vasomotor Symptoms Caused by Risk-Reducing Medications
Occasionally hot flashes or vasomotor symptoms can be brought on by external stimulation. Patients experiencing the symptoms should be counseled about attempting to avoid alcohol, caffeine, spicy foods, hot showers, and smoking, as these all can act as triggers.
Traditional medication treatment options for vasomotor symptoms and their possible side effects include gabapentin 300 mg daily with the ability to titrate up to 300 mg t.i.d.; clonidine 0.5 mg b.i.d. (side effect: hypotension); venlafaxine 37.5 mg up to 75 mg daily (side effect: dry mouth); and oxybutynin 15 mg daily (side effects: dry eyes, diarrhea, difficulty with urination).
Complimentary options for the treatment of vasomotor symptoms and hot flashes include acupuncture, Black Cohosh, Acteane/Estroven, and Relizen. Studies have demonstrated that lifestyle modification and a 30-minute session of acupuncture once a week are more effective in treating vasomotor symptoms than gabapentin or venlafaxine.
Uterine Cancer
Postmenopausal women with vaginal bleeding should undergo uterine ultrasound and possible endometrial biopsy. Premenopausal patients should undergo a uterine ultrasound.
Vaginal Atrophy/Dyspareunia
Over-the-counter oil- or water-based lubricants can be helpful. However, for women who are still symptomatic, low-dose vaginal hormone therapies can be considered. Estradiol cream or tablets, 100 mcg estradiol per gram of cream, 0.5 g of cream inserted intravaginally daily for 2 weeks and then inserted two to three times a week as necessary can be utilized to help counteract this side effect. There are also several vaginal rejuvenation therapies that are currently under study.
Cardiac Events
Tamoxifen and raloxifene are associated with a lower incidence of cardiac events. The aromatase inhibitors can lead to increases in cholesterol levels and should be monitored with yearly cholesterol and triglyceride panels.
Osteopenia/Osteoporosis
Osteopenia and osteoporosis are side effects associated with aromatase inhibitors. Any patient being prescribed at an aromatase inhibitor should be counseled on the importance of exercise and calcium and vitamin D supplementation for maintaining bone health. Individuals taking an aromatase inhibitor should have a baseline bone density (DEXA) scan performed prior to the start of the aromatase inhibitor and then yearly thereafter. Risk factors for osteopenia/osteoporosis include T score of less than 1.5, age greater than 65, low BMI (<20 kg/m 2 ), family history of hip fractures, personal history of fragility fractures, oral corticosteroid use for greater than 6 months, rheumatoid arthritis, and smoking (American Society of Breast Surgeons, 2019). Individuals exhibiting evidence of bone loss while taking an aromatase inhibitor for risk reduction should consider cessation of the medication.
Musculoskeletal Complaints/Joint Ache
This side effect is more common with aromatase inhibitors. It is thought that patients with low vitamin D levels may have a higher incidence of joint pain/achiness when taking the aromatase inhibitors. Therefore, it is important to make sure that a patient is taking vitamin D supplementation. Often these symptoms will resolve over time. However, patients can consider trying a different aromatase inhibitor to see if symptoms resolve. If symptoms do not resolve and the patient is experiencing significant difficulty, consider cessation of the medication.
Risk-Reducing Surgery
Risk-reducing mastectomy (RRM) is appropriate only for those women who are at very high risk for breast cancer. RRM is an appropriate option for individuals with pathogenic variants in high-penetrance genes, compelling family history, or prior thoracic radiation before the age of 30. While RRM has been considered in the past for individuals with a personal history of LCIS, the current preferred approach is risk-reducing therapy rather than surgical intervention. BRCA1/2 pathogenic variant carriers who undergo risk-reducing surgery have a substantially reduced breast cancer incidence and mortality. Appropriate preoperative counseling is essential to achieve appropriate postoperative expectations. RRM candidates have the option of reconstruction, including nipple-sparing mastectomy. Prior to surgical intervention, patients should undergo clinical breast exams, mammograms, and the possible screening breast MRI. Postoperatively individuals should undergo yearly clinical breast exams. After RRM there is no recommendation for screening mammogram, ultrasound, or MRI.
Management of the High-Risk Patient
For the purposes of this chapter, the classification and subsequent management of high-risk patient is as follows:
- 1.
Women with a history of mantle radiation between the ages of 10 and 30
- 2.
Women with a lifetime risk of breast cancer greater than 20% based on a history of classic lobular carcinoma in situ, ADH, or ALH
- 3.
Women with a lifetime risk greater than 20% defined by a model largely dependent on family history (i.e., Tyrer-Cuzick or Claus Model)
- 4.
Women over the age of 35 with a 5-year breast cancer risk greater than 1.66% based on the modified Gail model
- 5.
Women with a pedigree consistent with or having a known hereditary breast cancer syndrome
Women who underwent mantle radiation between the ages of 10 and 30 have an elevated risk for breast cancer secondary to the radiation alone. In the Late Effects Study Group Trial, the overall risk for breast cancer for women undergoing radiation at a young age was found to be 56-fold greater than the risk for breast cancer in the general population. Individuals should begin annual clinical encounters 8 years after completion of radiation. Annual screening mammograms with consideration for tomosynthesis should begin 8 years after completion of radiation therapy but not before age 30. These individuals can be recommended to begin screening breast MRI 8 years after completion of radiation therapy but not before age 25. For individuals that cannot undergo breast MRI, there is consideration for contrast-enhanced mammography or whole breast ultrasound. Patients should be counseled on lifestyle modification and risk-reducing strategies that were discussed earlier in the chapter.
Women with a personal history of classic-type lobular carcinoma in situ, ADH, or ALH should undergo a clinical breast encounter every 6 to 12 months starting at the time of diagnosis. Annual mammography with consideration for tomosynthesis should begin at the time of diagnosis of the high-risk lesion, but not before the age of 30. Patients may consider annual screening breast MRI starting at the time of diagnosis of the high-risk lesion, but not before age 25. The patient should be counseled in risk-reducing strategies such as a healthy lifestyle and risk-reducing agents.
For women with a lifetime risk of breast cancer greater than 20% based on family history models such as the Tyrer-Cuzick or Claus model, recommendations include a clinical breast exam every 6 to 12 months starting after age 21. Patients should consider annual mammograms starting 10 years prior to the age of the youngest family member with breast cancer, but not to begin before age 30. Patients may also consider an annual breast MRI, again starting 10 years younger than the age of the youngest family member as above, but not before the age of 25. Patients should be counseled regarding healthy lifestyle choices and risk-reducing medications.
Women with a calculated Gail Model 5-year risk greater than 1.66% are also recommended to consider a clinical breast exam every 6 to 12 months once they have been identified to be at increased risk. Individuals should consider annual mammography once they have been identified to be at high risk. There is not a recommendation for screening breast MRI based on Gail Model results. These individuals should also be counseled regarding healthy lifestyle and offered risk-reducing medications.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree