Fig. 1.1
The cost of DNA sequencing
The Role of Genetic Testing in the Clinic
The busy practitioner is challenged on a daily basis to recognize clinical scenarios that may be indicative of a genetic condition. This requires the recognition of clinical patterns, as well as taking a good family history. There are a number of reasons why genetic testing is important in the clinical setting (Table 1.1). An accurate diagnosis not only directs current management but also allows for a personalized road map for future surveillance for patients and presymptomatic family members. With the advent of prenatal and preimplantation diagnosis of genetic disorders, couples at risk for having children with heritable endocrine disorders may want to use genetic information to make reproductive decisions, and need appropriate counseling about the options available to them. Finally, with the flood of data coming in from genome-wide association studies (GWAS), and now with whole-exome and whole-genome sequencing, endocrinologists need to be ready for the demands of practicing individualized medicine for all of their patients.
Table 1.1
Clinical utility of genetic testing
• Accurate diagnosis and prognosis |
• Tailor management and surveillance for affected individuals |
• Identify presymptomatic individuals in affected families |
• Offer reproductive options to couples at risk |
To understand the significance of genetic test results, one has to first understand the importance of genetic variation in individuals. The sequence of nuclear DNA is nearly 99.9 % identical between any two humans [5]. Some DNA sequence differences have little or no effect on phenotype, whereas others are directly responsible for causing disease. Between these two extremes is the variation responsible for genetically determined phenotypic variability amongst individuals. Genetic disease is only the most obvious and extreme manifestation of genetic differences.
Gene Structure and Molecular Testing
There are approximately 25,000 genes in each of our cells. Even though the human genome has been sequenced, we still do not understand the functional significance of all of our genes. The coding regions of a gene (exons) are interspersed between large noncoding regions called introns (Fig. 1.2). Through the process of transcription and translation, the introns are spliced out to form messenger RNA (mRNA), which directs the formation of a specific protein. This process is influenced by noncoding regions such as promoters and enhancers, as well as regulatory regions upstream or downstream of the gene.
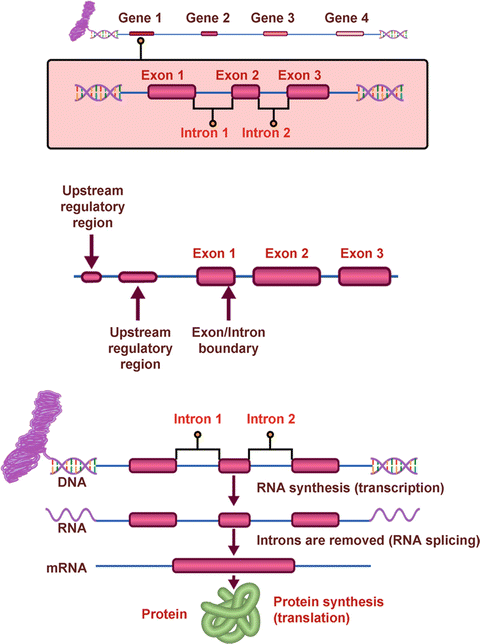

Fig. 1.2
Structure and function of genes
A mutation is simply a change in the nucleotide sequence of a gene. Most pathogenic mutations occur within exons, but they may also occur at the junction of introns and exons and thus affect splicing or, more rarely, occur in regulatory areas of the gene.
There are many different types of mutations, as illustrated in Fig. 1.3.
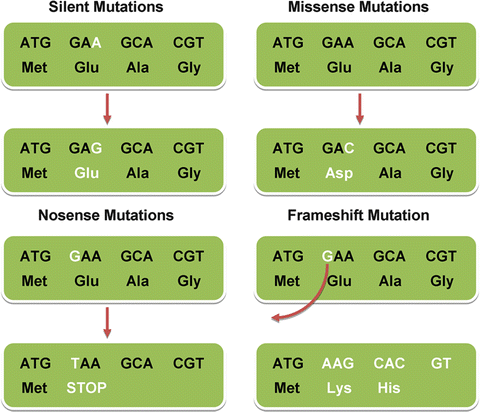

Fig. 1.3
Different types of point mutations
There are many different types of genetic tests (Table 1.2). These range from simple biochemical tests such as measuring 17 hydroxyprogesterone levels to diagnose 21 hydroxylase deficiency, or more complex tests such as a chromosome analysis (karyotype) to diagnose Turner syndrome (45,X) or Klinefelter syndrome (47,XXY). More recently, molecular karyotyping involving array comparative genomic hybridization (aCGH) has become the first-line test to diagnose chromosome microdeletion/microduplication disorders [6]. Molecular testing typically involves looking for sequence changes within a gene, and can be done in one of several ways. The simplest test involves targeted mutation analysis, attempting to identify a specific mutation at a specific position within a gene, obviating the need to sequence the entire gene. This is typically done when the disease-causing mutation is known in a family member (the proband), and other relatives at risk are being screened. In some disorders, only a few mutations are responsible for the disease, and thus mutation scanning can be performed, which involves searching for those select mutations only. In most diseases though, mutations are scattered throughout the gene, and thus sequencing of all exons and intron–exon junctions to look for defects that may cause splicing errors needs to be performed. For some disorders, a small percentage of mutations are known to occur in regulatory regions, and these regions can be sequenced as well. It is important to remember that even the most comprehensive sequencing test will not pick up large deletions or duplications within a gene, and thus techniques such as multiplex ligation probe amplification (MLPA) need to be performed in addition to sequencing.
Table 1.2
Types of genetic tests
• Biochemical testing |
• Karyotype (chromosome analysis) |
• Array comparative genomic hybridization (aCGH) |
• Linkage studies |
• Targeted mutation analysis |
• Mutation scanning |
• Sequencing |
• Deletion/duplication analysis |
• Genome-wide association studies |
• Whole-exome or -genome sequencing |
Once molecular testing has been performed, the results have to be interpreted with caution, as this can sometimes be quite a complex undertaking. The test may be positive for a known disease-causing mutation, unequivocally confirming the diagnosis, or negative for a mutation, making the suspected diagnosis unlikely, depending on the clinical sensitivity of the test for that particular disorder. Not infrequently though, equivocal results are seen, making interpretation difficult. A variation may be seen in the DNA sequence that has not been described previously as a mutation, and that is neither described as a benign polymorphism (an inconsequential change in the DNA sequence that is part of normal human variation). Such a change is typically characterized as a variant of unclear significance (VUS), and a number of questions have to be answered to determine the clinical significance of the variant (Table 1.3). This includes determining whether the variant in the DNA sequence leads to a change in the amino acid sequence of the protein, or is a “silent” change that encodes the same amino acid. It is important to remember though that a “silent” change may lead to alternative splicing and thus could be pathogenic. The nature of an amino acid change (polar vs. nonpolar, bulky vs. small) and the degree of conservation of an amino acid through different species are also important factors in determining whether a variant is truly pathogenic or not. Another important factor to consider is the frequency of the variant in apparently healthy individuals, but this has been challenging due to lack of appropriate representation of ethnic minorities in biobanks collecting such information. Despite multiple computations, it may still be difficult to establish whether the particular variant is associated with disease or not.
Table 1.3
Factors to consider when evaluating a variant of unclear significance
• Silent vs. missense variant
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



