GENETIC MANIPULATIONS IN ANIMALS IN VIVO
TRANSGENIC APPROACHES
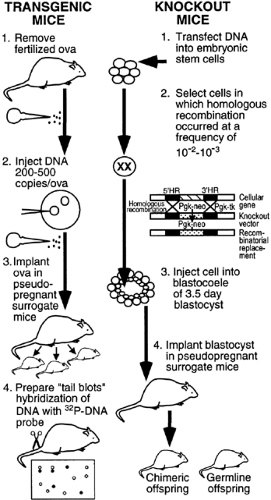
To create transgenic mice, DNA is injected into the male pronucleus of one-cell mammalian embryos (fertilized ova) that are then allowed to develop by insertion into the reproductive tract of pseudopregnant foster mothers (Fig. 2-3A). The transgenic animals that develop from this procedure contain the foreign DNA integrated into one or more of the host chromosomes at an early stage of embryo development. As a consequence, the foreign DNA is generally transmitted to the germline, and, in a number of instances, the foreign genes are expressed. Because the foreign DNA is injected at the one-cell stage, a good chance exists that the DNA will be distributed among all the progeny cells as development proceeds. This situation provides an opportunity to analyze and compare the qualitative and quantitative efficiencies of expression of the genes among various organs. The technique is quite efficient; >50% of postinjection ova produce viable offspring, and, of these, ˜10% efficiently carry the foreign genes. In the transgenic animals, the foreign genes can be passed on and expressed at high levels in subsequent generations of progeny.
Transgenic approaches can also be used to prevent the development of the lineage of a particular cell phenotype or to impair the expression of a selected gene. A cell lineage can be ablated by targeting a microinjected DNA containing a subunit of the diphtheria toxin to a particular cell type, using a promoter sequence specifically expressed in that cell type. The diphtheria toxin subunit inhibits protein synthesis when expressed in a cell, thereby killing the cell. The expression of a particular gene can be impaired by similar cell promoter– specific targeting of a DNA expression vector to a cell that produces an antisense mRNA to the mRNA expressed by the gene of interest. The antisense mRNA hybridizes to nuclear transcripts and processed mRNAs; this results in their degradation by double-stranded RNA–specific nucleases, thereby effectively attenuating the functional expression of the gene. The efficacy of the impairment of the mRNA can be enhanced by incorporating a ribozyme hammerhead sequence in the expressed antisense mRNA so as physically to cleave the mRNA to which it hybridizes. Another approach to producing a particular gene loss of function is to direct expression of a dominant negative protein (e.g., a receptor made deficient in intracellular signaling by an appropriate mutation, or a mutant transcription factor deficient in transactivation functions but sufficient for DNA binding). These dominant negative proteins compete for the essential functions of the wild-type proteins, resulting in a net loss of function.
Another approach, termed targeted transgenesis, combines targeted homologous recombination in embryonic stem (ES) cells with gain-of-function transgenic approaches.11,12,17 This method allows for targeted integration of a single-copy transgene to a single desired locus in the genome and thereby avoids problems of random and multiple-copy integrations, which may compromise faithful expression of the transgene in the conventional approach.
GENE ABLATION (KNOCK-OUTS)
A major advance beyond the gain-of-function transgenic mouse technique has been the development of methods for producing loss of function by targeted disruption or replacement of genes. This approach uses the techniques of homologous recombination in cultured pluripotential ES cells, which are then injected into mouse blastocysts and implanted into the uteri of pseudopregnant mice (Fig. 2-3B). The targeting vector contains a core replacement sequence consisting of an expressed-cell lethal-drug
resistance gene (selectable marker) (e.g., neomycin [Pgk-neo]) flanked by sequences homologous to the targeted cellular gene, and a second selectable marker gene (e.g., thymidine kinase [pgk-tk]). The ES cells are transfected with the gene-specific targeting vector. Cells that take up vector DNA and in which homologous recombination occurs are selected by their resistance to neomycin (positive selection). To select against random integration, a susceptibility to killing by thymidine kinase (negative selection) is used; only homologous recombination in which the thymidine kinase gene has been lost will confer survival benefit. Because the ES cells are injected into multicellular 3.5-day blastocysts, many of the offspring are mosaics, but some are germline heterozygous for the recombined gene. F1 generation mice are then bred to homozygosity so as to manifest the phenotype of the gene knock-out. Using this approach of targeted gene disruption, literally thousands of knock-out mice have been created. Many of these knock-out mice are models for human genetic disorders (e.g., those of endocrine systems such as pancreatic agenesis [homeodomain protein IDX-1], familial hypocalciuric hypercalcemia [calcium receptor], intrauterine growth retardation [insulinlike growth factor-II receptor], salt-sensitive hypertension [atrial natriuretic peptide], and obesity [α3-adrenergic receptor]).
resistance gene (selectable marker) (e.g., neomycin [Pgk-neo]) flanked by sequences homologous to the targeted cellular gene, and a second selectable marker gene (e.g., thymidine kinase [pgk-tk]). The ES cells are transfected with the gene-specific targeting vector. Cells that take up vector DNA and in which homologous recombination occurs are selected by their resistance to neomycin (positive selection). To select against random integration, a susceptibility to killing by thymidine kinase (negative selection) is used; only homologous recombination in which the thymidine kinase gene has been lost will confer survival benefit. Because the ES cells are injected into multicellular 3.5-day blastocysts, many of the offspring are mosaics, but some are germline heterozygous for the recombined gene. F1 generation mice are then bred to homozygosity so as to manifest the phenotype of the gene knock-out. Using this approach of targeted gene disruption, literally thousands of knock-out mice have been created. Many of these knock-out mice are models for human genetic disorders (e.g., those of endocrine systems such as pancreatic agenesis [homeodomain protein IDX-1], familial hypocalciuric hypercalcemia [calcium receptor], intrauterine growth retardation [insulinlike growth factor-II receptor], salt-sensitive hypertension [atrial natriuretic peptide], and obesity [α3-adrenergic receptor]).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree