GENETIC CONTROL OF SEX DIFFERENTIATION
The X and Y sex chromosomes and the autosomes contain loci that must remain intact for normal sexual development. That
autosomal factors influence sexual development is potentially important for clinical management. For example, an autosomal translocation may result in genital abnormalities. Before the various disorders are formally considered, summarizing the genetic control of sex differentiation may be helpful.
autosomal factors influence sexual development is potentially important for clinical management. For example, an autosomal translocation may result in genital abnormalities. Before the various disorders are formally considered, summarizing the genetic control of sex differentiation may be helpful.
TESTICULAR DEVELOPMENT
Consideration of individuals with various abnormalities of the X and Y chromosomes led to the concept that the Y chromosome carries genetic information required for testis formation.7 Such a hypothetical gene was named testis-determining factor (TDF) and was postulated to be present in males and absent in females. In that sense, TDF is the sex-determining gene, although many other genes are required for the formation of a testis, and these are distributed among the autosomes and the X chromosome. Several experiments have resulted in the identification and cloning of a gene located on the Y chromosome called the sex-determining region Y (SRY) gene that appears identical to the conceptual TDF gene.8
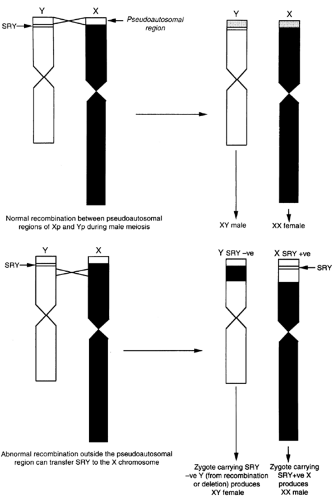
SRY was identified and cloned by constructing maps of the Y chromosome. The Y chromosome is composed of two parts with different functions. One part consists of the two pseudoautosomal regions that are found on the X and Y chromosomes and are required for correct pairing and segregation during meiosis.9,10 The major pseudoautosomal region is located at the end of the short arm of each sex chromosome. During each male meiosis, a single recombination occurs between the X and Y chromosomes in this pseudoautosomal region (Fig. 90-6), allowing the construction of recombination maps of the terminal part of the short arms of the sex chromosomes.11 (A second pseudoautosomal region exists at the ends of the long arm of the sex chromosomes.) The second part of the Y chromosome includes TDF and is not shared with the X chromosome. Because the Y-specific region does not normally recombine with the X chromosome, it is not amenable to classic genetic analysis. Study of the genomes of XX males, however, in which it was proposed that Y-derived sequences would be present on the X chromosome as a result of an abnormal recombination between the unique portions of the sex chromosomes during male meiosis, has allowed the construction of maps of the short arm of the Y chromosome.12,13 TDF was mapped to the most distal part (i.e., farthest from the centromere) of the unique portion of the short arm of the Y chromosome.
SRY is a single-copy gene located in the smallest Y chromosomal region capable of inducing sex reversal. It is expressed in the genital ridge only during the appropriate time of embryonic development when testicular cords form.8 Moreover, it is deleted or mutated in cases of human XY females, and the presence of the equivalent gene in the mouse, called SRY, can sex-reverse XX mice into males.8,14,15,16 and 17 The protein product of SRY contains an 80-amino-acid domain with a motif shared by a high-mobility group(HMG) of transcription factors that bind to DNA and are important in the regulation of gene transcription. Studies of the DNA-binding properties of the SRY HMG box of proteins in the promoter regions of the P450 aromatase gene (which converts testosterone to estradiol and is down-regulated in the male embryo) and of the antimüllerian hormone (AMH) gene (which is responsible for regression of the müllerian ducts) support the hypothesis that SRY directly regulates male development
through sequence-specific regulation of target genes.8,18,19 Other target genes remain to be identified.19a Active SRY participation in morphogenesis appears to be required for formation of a testis from the bipotential genital ridge.
through sequence-specific regulation of target genes.8,18,19 Other target genes remain to be identified.19a Active SRY participation in morphogenesis appears to be required for formation of a testis from the bipotential genital ridge.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree