General Principles of Antimicrobial Selection: Introduction
When treating infection in older patients, selection of an antimicrobial regimen often precedes the identification of the causative pathogen and may sometimes be necessary even before the specific site of infection has been established. In these cases, the choice of therapy is typically based on the clinician’s estimation of the most likely causative organism as well as properties of the available antimicrobial agents. This method of antimicrobial selection is described as “empiric,” as opposed to “pathogen-directed,” in which the causative organism has already been determined by the results of clinical cultures and antimicrobial susceptibility testing. This chapter addresses the unique challenges of selecting optimal empiric antimicrobial therapy for older patients with known or suspected infection.
To provide both a framework for discussion as well as a practical tool to be applied at the bedside, the process of selecting empiric antimicrobial therapy for older patients may be considered in three steps. First, to determine the potential causative pathogen as well as the appropriateness of initiating antimicrobial therapy, clinical and epidemiological clues are examined in order to establish the most likely site of infection. Next, estimation of the likelihood of antimicrobial resistance, together with consideration of the seriousness of the patient’s infection and overall clinical status are used to determine the most appropriate breadth of antimicrobial coverage. Finally, pharmacological issues, including the pharmacokinetics of the available agents, potential toxicities and drug interactions are considered in order to facilitate the final selection of the most appropriate antimicrobial agent or agents. Once empiric therapy is initiated, the patient’s clinical progress is closely followed and the antimicrobial regimen is broadened or narrowed according to the results of testing in the clinical microbiology laboratory. This process of choosing empiric therapy is summarized in Table 125-1.
I. Estimate the most likely causative pathogen if not known
|
II. Determine the appropriate breadth of initial antimicrobial therapy
|
III. Integrate pharmacological data to select the empiric choice of therapy
|
IV. Monitor clinical course and laboratory data to refine and narrow antimicrobial regimen
|
In the section that follows, this systematic approach to the management of older patients with known or suspected infection is reviewed in greater detail. Thereafter, the strategy is applied to the discussion of specific recommendations for antimicrobial therapy for a variety of infectious syndromes.
When evaluating any patient with possible infection, determination of the most likely site of infection is a critical first step for the clinician who wishes to establish the causative pathogen in order to select the most appropriate antimicrobial regimen. The unique and often enigmatic manifestations of infection in older patients serve to complicate this endeavor. An understanding of the physiological factors that influence the clinical presentation of infection in older patients provides clinicians caring for this group with the necessary tools and increased sensitivity to detect evidence of infection in these vulnerable patients and lower the threshold to initiate empiric therapy even when only subtle signs or symptoms of infection are present.
The factors that increase vulnerability to infection and those that result in atypical or blunted manifestation of infection are often the very same in the elderly patient. These issues are addressed in greater detail in Chapter 124.
Once clinical clues have raised the suspicion of infection and the decision has been made to initiate empiric antimicrobial therapy, epidemiological factors may offer additional information as to the most likely etiological agent. For example, a history of recent hospitalization or long-term care facility residence should increase concern for a wider diversity of infecting strains. For patients with this history, the relatively innocuous gram-positive flora that typically resides on the skin may be replaced by more aggressive gram-negative species that are more likely to infect surgical wounds, intravascular catheters and the urinary tract.
When considering epidemiological clues, knowledge of local outbreaks of infection should inform the diagnostic evaluation of a new case of illness in an individual patient. Seasonal factors should also be considered. For example, influenza may be an exceptionally common cause of respiratory infection from October to March, whereas other causes are more likely at other times of the year. The known geographic distribution of certain pathogens and infectious syndromes is also important for the clinician to keep in mind. The pathogen responsible for fever in a traveler returning from the Amazon Basin (malaria) is apt to be different than the organism causing the same presentation in an elderly resident of the desert Southwest (coccidiomycosis) or Martha’s Vineyard (babesiosis).
Following the identification of the site of infection and determination of the most likely causative organism, the next step in the management of the infected older patient is an evaluation of the severity of infection. While an accurate assessment of the severity of infection will inform a clinician’s decision as to whether or not to start empiric therapy, the same information should influence the breadth of antimicrobial coverage once therapy is initiated. In general, the most appropriate response to evidence of a severe infection in an older patient on the part of the treating clinician should be rapid administration of one or more potent antimicrobial agents with efficacy against the most likely causative pathogen. There is evidence that rapid delivery of the first dose of therapy is particularly critical for the treatment of serious infections in older patients, such as sepsis, meningitis and pneumonia.
The severity of infection not only influences the choice of antimicrobial regimen, but may also influence antimicrobial dosing and the route of delivery selected. In all cases of severe infection, the goal is to ensure delivery of an adequate level of the antimicrobial agent to the site of infection in a sufficient concentration that will result in the killing of the pathogen. In general, parenteral administration of antimicrobial agents must be employed for serious infections. Parenteral therapy may not be necessary for the entire duration of treatment, but should at least be used until the patient has demonstrated some response to therapy, such as resolution of fever or normalization of leukocytosis.
In general, use of an agent with a broad antimicrobial spectrum may be most appropriate in the early stages of treating a serious infection in an older patient. This should ensure that the agent is active against the pathogen, the identity of which is usually unknown at the time that therapy is initiated. Once the causative pathogen is identified, an agent with a narrower spectrum of activity can be used to more specifically target the pathogen without the attendant consequences of broad-spectrum therapy, such as disruption of the normal bacterial flora and selection of pathogens with antimicrobial resistance.
In addition to considering the severity of infection, the clinician pursuing a rational approach to antimicrobial selection for an older patient will incorporate the likelihood of antimicrobial resistance into their thinking. This is particularly an issue for patients in long-term care facilities or acute care hospitals where transmission of pathogens between patients is a particular concern. The high rates of antimicrobial use in long-term care and acute care facilities also leads to the selection of antimicrobial-resistant organisms. Inappropriate prescription of antimicrobials, reported to approach fifty percent of prescriptions in these settings, is a major contributor to the continued escalation of this problem.
In all cases of known or suspected infection with antimicrobial-resistant pathogens, knowledge of local susceptibility profiles for common pathogens should inform all decisions about antimicrobial choice. Institutions and laboratories should be encouraged to provide data on local susceptibilities to clinicians. Consideration should be given to an antimicrobial regimen with a broader spectrum of coverage until the causative pathogen is identified.
When infection with an antimicrobial-resistant pathogen is anticipated, it may be appropriate to consider the use of combination therapy—the concurrent use of two or more agents from different antibacterial classes. The rationale for the use of combination therapy is to increase the likelihood that the administered antibacterials are effective against the known or suspected pathogen, whether the increase in antimicrobial activity of the combination is additive or synergistic. The decision to initiate combination therapy must be weighed against the possibility of increased toxicity, additional expense, and further selection of resistant organisms. In general, while combination therapy may be appropriate for empiric therapy for a seriously ill older patient, the regimen can generally be narrowed to a single effective agent once a pathogen is isolated, identified and susceptibility results are available.
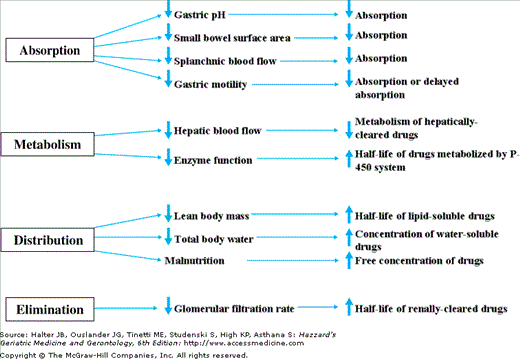
Having established the need for empiric antimicrobial therapy and the most likely causative pathogen through careful examination of clinical and epidemiological clues and after considering the patient’s severity of illness and the likelihood of antimicrobial resistance to determine the appropriate breadth of coverage, the clinician treating the infected older patient may still have a number of therapeutic options from which to choose. In making a final decision regarding the optimal antimicrobial regimen, he/she will need to draw upon an up to date knowledge of pharmacological principles, especially as they apply to this vulnerable population. While the recommended antimicrobial agent and dosing schedule for a particular infection should apply to most populations, there are unique pharmacological issues that affect the choice of therapy for older patients (Figure 125-1).
Theoretically, oral drug absorption may be reduced by physiological changes common in elderly populations, such as elevated gastric pH or reduced splanchnic blood flow. It is more likely, however, that coadministered medications are the primary culprits that interfere with the absorption of certain antimicrobial agents in this population. It may be appropriate to review timing of administration of medications such as antacids when a new antimicrobial agent is prescribed. While administration of parenteral therapy should avoid potential problems with oral absorption, the increased risk of intravascular catheter infection in this population must be weighed against the benefits of this approach.
Once absorbed, drug metabolism may be affected in the older adults. Age-related changes in hepatic function, such as reduced hepatic blood flow, smaller hepatic mass, and reduced enzyme function may lead to inefficient drug metabolism, which, in general, slows drug clearance. Patients prescribed other medications that interfere with antimicrobial metabolism may experience either higher or lower levels of antimicrobial than anticipated.
In many older patients, the volume of distribution of medications is altered. This can be because of the reduction in lean body mass, increase in proportion of adipose tissue, or reduction in total body water. Changes in protein-binding caused by malnourishment can also affect volume of distribution. These changes can lead to unpredictability in medication levels and are particularly important to keep in mind when choosing loading doses of antimicrobial agents.
Geriatric patients may be more prone to certain medication toxicities because of the vulnerability of the end organ. For example, exacerbation of hearing loss or impairment of the vestibular system is more likely to occur in geriatric patients treated with aminoglycosides. Renal impairment is of particular concern following aminoglycoside therapy, especially in older patients with pre-existing renal dysfunction. A list of frequently encountered adverse effects associated with specific antimicrobial agents is provided in Table 125-2.
ANTIMICROBIAL CLASS/AGENT | INTERACTING DRUGS | ADVERSE EFFECT(S) |
|---|---|---|
Acyclovir | Aminoglycosides | Increased nephrotoxicity/neurotoxicity |
Narcotics | Increased meperidine effect | |
Aminoglycosides | Acyclovir | Inreased nephrotoxicity/neurotoxicity |
Oral anticoagulants | Potentiation of anticoagulation effects | |
Bumetanide | Increased ototoxicity | |
Cephalosporins | Increased nephrotoxicity | |
Cyclosporine | Increased nephrotoxicity | |
Digoxin | Decreased digoxin effect (oral aminoglycosides only) | |
Furosemide | Increased nephrotoxicity/ototoxicity | |
Magnesium sulfate | Increased neuromuscular blockade | |
Vancomycin | Possible increased nephrotoxicity and ototoxicity | |
Azithromycin | Aluminum/magnesium antacids | Decreased peak concentrations |
Digoxin | Increased digoxin concentrations | |
Cephalosporins | Aminoglycosides | Increased nephrotoxicity |
Furosemide | Increased nephrotoxicity | |
Clarithromycin | Oral anticoagulants | Potentiation of hypoprothrombinemia |
Benzodiazepines | Increased CNS toxicity | |
Carbamazepine | Increased carbamazepine toxicity | |
Corticosteroids | Increased effect and possible toxicity of methylprednisone | |
Cyclosporine | Increased cyclosporine toxicity | |
Digoxin | Increased digoxin concentrations | |
Phenytoin | Possible increased or decreased effect | |
Rifampin | Decreased clarithromycin concentrations | |
Erythromycin | Oral anticoagulants | Potentiation of hypoprothrombinemia |
Benzodiazepines | Increased CNS toxicity | |
Carbamazepine | Increased carbamazepine toxicity | |
Corticosteroids | Increased effect and possible toxicity of methylprednisone | |
Cyclosporine | Increased cyclosporine toxicity | |
Digoxin | Increased digoxin concentrations | |
HMG-CoA reductase inhibitors | Increased risk of rhabdomyolysis | |
Loratadine | Increased loratadine toxicity | |
Phenytoin | Possible increased or decreased effect | |
Valproic acid | Increased valproic acid toxicity | |
Fluconazole | Benzodiazepines | Increased CNS toxicity |
Coumarin anticoagulants | Increased prothrombin times | |
Cyclosporine | Increased cyclosporine concentrations | |
HMG-CoA reductase inhibitors | Increased risk of rhabdomyolysis | |
Phenytoin | Increased phenytoin concentrations | |
Rifampin | Increased fluconazole concentrations | |
Sulfonylureas | Increased plasma concentrations and decreased metabolism of sulfonylureas | |
Thiazides | Increased fluconazole concentrations | |
Fluoroquinolones | Antacids | Decreased fluoroquinolone effect |
Oral anticoagulants | Prolonged prothrombin times | |
Cyclosporine | Increased risk of nephrotoxicity; increased serum cyclosporine concentrations | |
Iron | Decreased serum fluoroquinolone concentrations | |
NSAIDs | Possible increased risk of CNS stimulation | |
Sucralfate | Decreased serum fluoroquinolone concentrations | |
Linezolid | SSRIs | Increased risk of serotonin syndrome |
Metronidazole | Oral anticoagulants | Increased anticoagulant effect |
Nitrofurantoin | Antacids | Possible decreased nitrofurantoin effect |
Fluoroquinolones | In vitro antagonism of quinolone activity | |
Rifampin (and other rifamycins) | Analgesics | Possible decreased concentrations and activity |
Oral anticoagulants | Possible decreased concentrations and activity | |
Anticonvulsants | Possible decreased concentrations and activity | |
Beta-blockers | Possible decreased concentrations and activity | |
Clarithromycin | Decreased clarithromycin concentrations/increased rifamycin toxicity | |
Corticosteroids | Possible decreased concentrations and activity | |
Diazepam | Possible decreased concentrations and activity | |
Digoxin | Decreased digoxin effect | |
Fluconazole | Decreased fluconazole effect | |
HMG-CoA reductase inhibitors | Decreased HMG effect | |
Isoniazid | Possible increased hepatotoxicity | |
Narcotics | Possible decreased concentrations and activity | |
Verapamil | Possible decreased concentrations and activity | |
Sulfonamides | Oral anticoagulants | Increased anticoagulant e ffect |
Hypoglycemics | Increased hypoglycemic effect | |
Tetracyclines | Antacids | Decreased oral tetracycline effects |
Oral anticoagulants | Increased anticoagulant e ffect | |
Bismuth subsalicylate | Decreased oral tetracycline effects | |
Carbamazepine | Decreased doxycycline effect | |
Digoxin | Increased digoxin effect | |
Lithium | Increased lithium toxicity | |
Phenytoin | Decreased doxycycline effect | |
Rifampin | Possible decreased doxycycline effect | |
Vancomycin | Aminoglycosides | Possible increased nephrotoxicity and ototoxicity |
The problem of polypharmacy in the geriatric population is thoroughly reviewed in Chapter 72. It is appropriate here, however, to consider the contribution of antimicrobial agents to this problem. In some respects, the individuals most susceptible to polypharmacy are also those at greatest risk for infection—frail older adults with multiple comorbid conditions. Drug–drug interactions are of primary concern when adding a new prescription for an antimicrobial to an already long medication list. A comprehensive list of potential interactions is presented in Table 125-3.
ANTIMICROBIAL CLASS/AGENT | COMMON ADVERSE EFFECTS |
|---|---|
Acyclovir | Nephrotoxicity, CNS toxicity |
Amantadine/rimantadine | CNS toxicity |
Aminoglycosides | Nephrotoxicity, ototoxicity, vestibular toxicity |
Azithromycin | GI toxicity (diarrhea, nausea, vomitting, abdominal pain), candidiasis/vaginitis |
Beta-lactam agents | Rash, hypersensitivity reactions, bone marrow toxicity, CNS toxicity (seizure), interstitial nephritis, antibiotic-associated diarrhea, C. difficile-associated diarrhea |
Clarithromycin | GI toxicity (diarrhea, nausea, vomitting, abdominal pain), CNS toxicity |
Clindamycin | GI toxicity (esophagitis, gastritis, nausea, vomitting, abdominal pain), antibioticassociated diarrhea, C. difficile-associated diarrhea |
Erythromycin | GI toxicity (diarrhea), hepatotoxicity, QT prolongation, torsade de pointes, ototoxicity |
Fluconazole | Hepatotoxicity |
Fluoroquinolones | QT prolongation, torsade de pointes, CNS toxicity, peripheral neuropathy, tendone rupture, C. difficile-associated diarrhea |
Isoniazid | Hepatotoxicity, peripheral neuropathy |
Linezolid | Bone marrow suppression (thrombocytopenia), GI toxicity (nausea, vomitting, diarrhea), headache, peripheral neuropathy |
Metronidazole | Dysgeusia, anorexia, GI toxicity (nausea, vomitting), CNS toxicity (dizziness, lightheadedness, headache), peripheral neuropathy, disulfram-like effects when alcohol is ingested |
Nitrofurantoin | Pulmonary infiltrates, peripheral neuropathy |
Rifampin |