a diet poor in fruits and vegetables19
the consumption of maté, a stimulant beverage commonly consumed in South America20
the chewing of betel quid, a stimulant preparation commonly used in parts of Asia.21
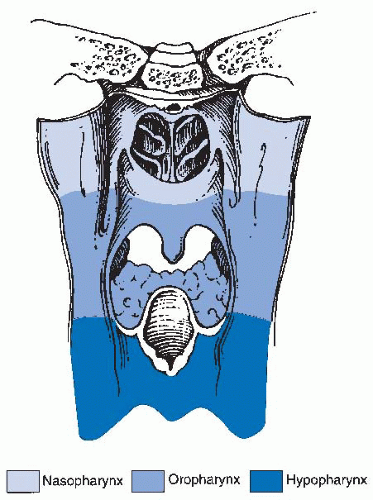
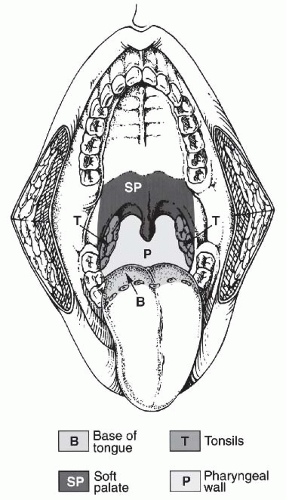
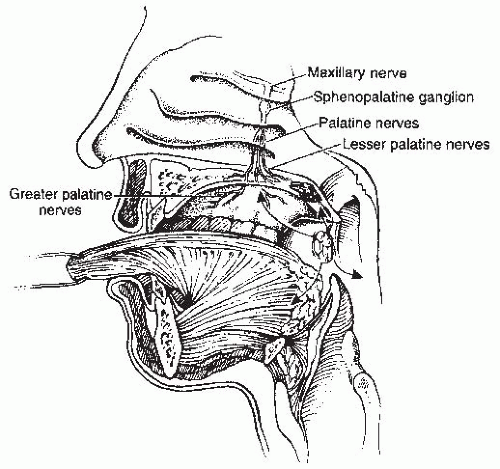
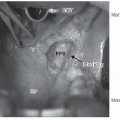
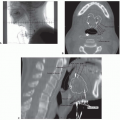
inferiorly. It is continuous with the oral cavity anteriorly and communicates with the nasopharynx above and the supraglottic larynx and the hypopharynx below. Within the oropharynx are four different sites: soft palate, tonsillar region (fossa and pillars), BOT, and posterior and lateral oropharyngeal wall between the nasopharynx and the pharyngoepiglottic fold (Fig. 17-2).
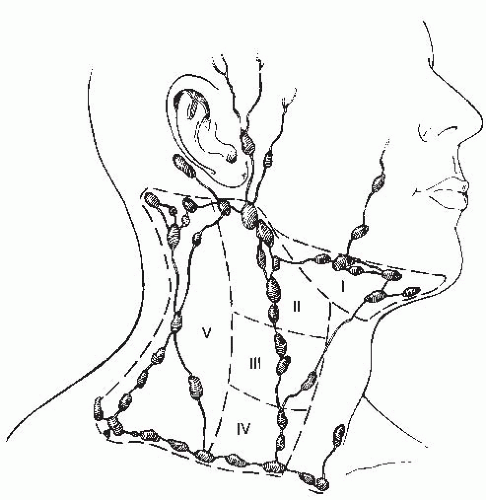
TABLE 17.1 Percentage Incidence of Cervical Lymph Node Metastasis as Determined by Clinical Examination | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
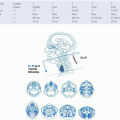
TABLE 17.2 Defining the Risk of Involvement for Each Neck Nodal Level in Patients with Early T-Stage/Node-Positive Human Papillomavirus-related Oropharyngeal Carcinoma | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
TABLE 17.3 Percentage Incidence of Cervical Lymph Node Metastasis in Soft Palate Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 17.4 Differential Diagnosis of an Oropharyngeal Mass | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
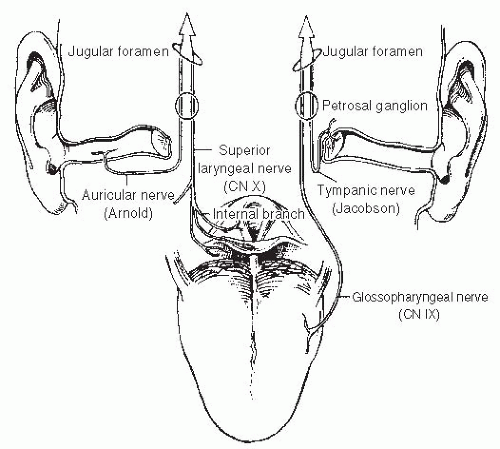
Patients may experience the sensation of a mass or discomfort in the throat, with bleeding and pain at later stages. Patients might also experience difficulty with speech and swallowing. Occasionally, referred otalgia is the first symptom.
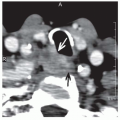
the tonsil or the lateral pharyngeal wall, the examiner should test for anesthesia in the distribution of the ipsilateral mandibular nerve (V3). Any abnormality might suggest involvement of the inferior alveolar nerve in its pathway as it courses through the mandible or the base of skull and may direct the appropriate imaging study.56 Because the tonsil is adjacent to the ascending ramus of the mandible, the BOT, and the parapharyngeal space, tumors may extend into these areas. This can often be detected by palpation. Indirect mirror examination and flexible endoscopy are important parts of the physical examination. It is useful to take a photograph or extensively diagram the physical findings in every patient. This can include a videotape. Such records are an excellent means of documenting the physical findings and of comparing future examination results to the initial presentation. In selected cases, an examination under anesthesia is recommended as a mean of obtaining information that is not completely accessible during office examination.
TABLE 17.5 American Joint Committee on Cancer (AJCC) Classification of Oropharyngeal Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
better for those patients with HPV-positive disease regardless of the treatment modality.1 Although HPV/p16-positive patients have better outcomes than HPV/p16-negative patients, this advantage disappears in the HIV-positive population and heavy smokers. Mourad et al. reported on 73 HIV-positive patients with SCCHN, of whom 24 patients had OPC (12 of whom were HPV/p16 positive).62 The outcomes revealed no difference between HPV/p16-positive versus HPV/p16-negative in the setting of HIV-positive patients and no advantage to chemo-RT over RT alone. These data are limited by their retrospective nature, but suggest that HIV status is an important prognostic factor.63 Gillison et al. reported that the risk of OPC progression and death increases directly as a function of tobacco exposure at diagnosis and during therapy and is independent of tumor p16 status and treatment.8 Smoking-induced OPC has been associated with mutated p53, epidermal growth factor receptor (EGFR) overexpression, and lower HPV/p16 expression.64 Thus, given its unique prognostic significance, HPV is reviewed in detail in Chapter 12. In addition, Chapter 3 reviews all other important prognostic factors.
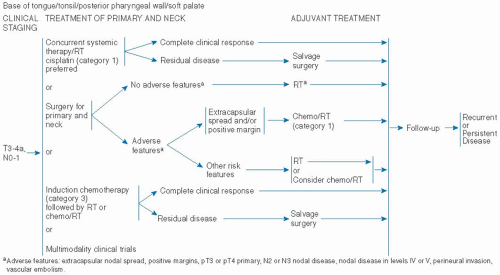
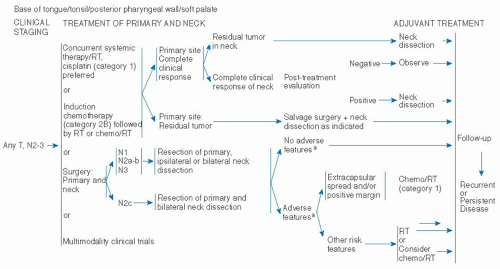
be attractive to certain patients. These complex issues require the patient to have a thorough understanding of all the options and management algorithms, so that a personalized choice can be made. Multidisciplinary evaluation and discussion is mandatory.
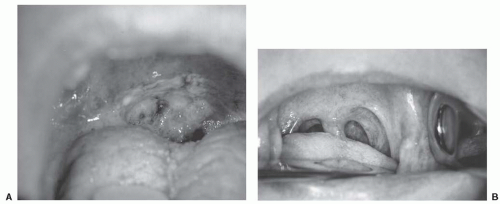
primary RT. Stage I disease included 23 patients (16%) and stage II disease included 36 patients (25%). The 5-year initial and ultimate (i.e., after successful salvage) LRC rates for stage I were 84% and 89%, respectively. The 5-year initial and ultimate LRC rates for stage II were 85% and 88%, respectively. Specifically, the LC rates for both T1 and T2 were 90%. Nodal control rate at 5 years for N0 disease was 90%. The 5-year freedom from distant metastases rate was 95% for stage I and 97% for stage II. The 5-year DSS for stages I and II were 89% and 87%, respectively.97 Figure 17-11 shows a T2 SCC of the soft palate before and after RT.
metastases. Patients with early disease (n = 90) had a DSS of 79%, whereas T1-2 but with neck-positive nodes (n = 22) had a DSS of 56%. For OS and DSS, N classification was predictive of outcome. For LC and DC, margin status was a significant predictor, whereas the T classification was only a significant predictor for LC.98
TABLE 17.6 Five-year Oncologic Outcomes of Primary Surgery ± Postoperative Radiation Therapy in Soft Palate Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
T1-2 tumors of which 70% with N0. At a median follow-up of 5.7 years, none of the patients with T1 or N0 lesions had experienced contralateral neck failure. The median 3-year LC rate for stages I and II were 91% and 76%, respectively. The 3-year median ipsilateral RC for stages I and II were 98% and 91%, respectively. For the whole cohort, the 3-year cause-specific survival was 76%. The only patients subsets with significant risk of contralateral neck failure (>10%) were those with tumor involvement of the medial one-third of palate or BOT.116
At a median follow-up of 5.7 years, the median 3-year LC for stages III and IVA/B were 79% and 70%, respectively. The 3-year median RC for stages III and IVA/B were 75% and 60%, respectively. Contralateral neck failure occurred in 3%.116
TABLE 17.7 Oncologic and Functional Outcomes of OPC, Tonsil, and Base of Tongue, Treated by Primary RT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree