General Principles and Management
Wayne M. Koch
Edward Stafford
Christine Chung
Harry Quon
INTRODUCTION: INCIDENCE, ETIOLOGY, AND EPIDEMIOLOGY
Cancer of the oral cavity is actually a collection of distinct and overlapping disorders with differing etiology, clinical manifestation, and treatment. Tumors are most commonly categorized by anatomical location and histologic diagnosis. The quintessential oral cancer arises in an individual who has used tobacco and alcohol. These lesions tend to occur on the lateral or ventral tongue, the floor of mouth (FOM), or the alveolar ridge, most often in men between the ages of 50 and 70 years, and are differentiated squamous cell carcinomas (SCC). However, there is increasing awareness that nonsmokers also can develop oral SCC (OSCC), most often on the lateral tongue but also elsewhere including the buccal mucosa and the alveolar ridge, often in younger and older patients, including disproportionately more women. Patients with lip cancer also have distinct demographic and clinical patterns, whereas minor salivary gland cancers are much less common and occur without known risk profile. These distinct categories support the hypothesis that the underlying process of tumorigenesis is also quite different among the broad cohort of patients with oral cancer.
Oral cancer is potentially a devastating disease. At late stages, it is very difficult to control and can result in a miserable demise. Even when detected early, treatment necessarily brings collateral damage to a region of the body that has great functional importance for communication and deglutition as well as cosmetic and therefore psycho-social impact.
According to the 2012 Cancer Facts and Figures of the American Cancer Society, there will be an estimated 26,740 cases of cancer of the oral cavity including tongue, mouth, and “other” regions in the United States in 2012. One-third of these cases will arise in women. During the same year, there will be 5,520 American deaths due to these cancers. Oral cavity and oropharyngeal cancers together comprise the eighth most common cancer in American men (3 % of cancers). The rate of oral cancer declined by 1 % per year in American women from 2004 to 2008, and the death rate for oral cavity and oropharynx cancers has declined over 30 years by 1.2% in men and 2.2% in women. This latter statistical observation may be partly due to a proportional increase in human papillomavirus (HPV)-related oropharyngeal cancers included in data collection, a subpopulation with markedly improved response to therapy that is not seen for cancers of the oral cavity. Survival rates for African Americans with oral cancer remains well behind that of White Americans (45 % 5-year survival vs. 65%).1
Worldwide, the American Cancer Society estimates that oral cavity cancer is the 10th most common cancer among men with 170,900 cases estimated in 2008 (the last year with data available). Most of these cases were expected to be from developing countries (107,700), and most of those cases result in cancer-related death (61,200).2 In South Central Asia and Melanesia, oral cancer is the second most common cancer among adult males.
Using the Surveillance, Epidemiology, and End Results (SEER) database, from 1998 to 2001, Piccirillo and coauthors have calculated relative survival for patients with oral cancer. Relative survival is defined as the ratio of observed survival divided by the survival that would be expected for a cancer-free individual from the same age, gender, and racial group. These data are derived from >40,000 individuals with head and neck cancer living in 12 geographic areas of the United States including 8,637 cases of tongue cancer (base of tongue and oral tongue combined), 3,286 cases of FOM cancer, and 5,946 cases of “gum and other mouth” cancers. The 5-year relative survival rate was found to be 53.1% for tongue, 59.5% for gum, and 52.7% for FOM tumors. Broken down by gender and race, survival is slightly better for women and markedly poorer for black than for white patients in all three anatomic groups. Outcome varies with stage of cancer declining from 70% to 96% 5-year survival for stage I oral cancer to 26% to 48% for stage IV cancers (Tables 16.1). Survival for oral tongue compared to base of tongue cancer is better for small tumors (75.9% for stage I oral tongue 5-year relative survival
compared to 51.5% for stage I base of tongue lesions) but worse for all later stages (26.5% relative 5-year survival for stage IV oral tongue compared to 41.9% for stage IV base of tongue).3 Survival of oral cancer patients also varies widely in different parts of the world. According to American Cancer Society Global statistics, 5-year relative survival for oral cancer patients is highest in the United States and Germany (60.7%) and lowest in Poland, Thailand, and India (36%-37%).2
compared to 51.5% for stage I base of tongue lesions) but worse for all later stages (26.5% relative 5-year survival for stage IV oral tongue compared to 41.9% for stage IV base of tongue).3 Survival of oral cancer patients also varies widely in different parts of the world. According to American Cancer Society Global statistics, 5-year relative survival for oral cancer patients is highest in the United States and Germany (60.7%) and lowest in Poland, Thailand, and India (36%-37%).2
TABLE 16.1 Five-Year Relative Survival (%) of Oral Cancer from SEER Database3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cancer of the lip has a distinctive demographic pattern in the United States. It affects principally white men, who account for 80% of cases in the published SEER database analysis of 12 regions.3 Lip carcinoma accounts for roughly 12% of all noncutaneous head and neck cancers. White women make up another 17.8 % with African Americans accounting for only 1.1% of cases. The vast majority of cases are diagnosed and treated at an early stage (83.2% stage I), accounting for an overall excellent outcome (93.5% relative 5-year survival). This is the lowest disease-related mortality among oral cancers. The incidence is reported to be high in Australia and New Zealand (10.2 per 100,000 men).4 More than 90% of carcinomas of the lip involve the lower lip, attributed to sun exposure. The upper lip (2%-7%) and commissure (<1%) are less frequently involved. A variety of malignant histologies arise in the lip, most commonly basal cell and squamous cell carcinoma and less common lesions such as minor salivary gland tumors, melanoma, and sarcoma.
Cancer of the hard palate accounts for 10% of the neoplasms of the oral cavity. There is a great variety of malignant neoplasms that arise in the hard palate, including tumors of epithelial, salivary, hematopoietic, and mesenchymal tissues. SCC comprise only about 50% of these tumors. Carcinomas originating from minor salivary glands are the most common non-squamous cell malignancies in this region. Inexplicably, salivary cancers typically arise near the foramen of the descending palatine artery near the second molar tooth and the hard palate-soft palate junction. Less common malignancies presenting in the hard palate include hematologic malignancies such as lymphoma and plasmacytoma, polymorphic reticulosis (or lethal midline granuloma), Kaposi sarcoma, and mucosal melanoma.
Risk Factors for OSCC
The use of alcohol and tobacco account for approximately 75 % of all cases of OSCC worldwide.5 It is widely agreed that tobacco plays the major role in this risk profile. Poor oral hygiene and nutritional deficits are also invoked, but because these factors often coexist with alcohol and tobacco abuse, their relative contribution is difficult to assess. In a large pooled case-control international study, smoking alone, among alcohol abstainers, was associated with a relative risk of over threefold for the development of OSCC in Europe and South America, with a somewhat lower risk estimated for persons from South Asia and North America.6 These differences may reflect the level of carcinogens in tobacco products sold in different regions. The risk of cancer increased with exposure measured in pack years from about 1.5-fold for light smokers (<10 pack years) peaking at fourfold for those individuals who have >30 pack years of smoking. Alcohol alone in persons who do not use tobacco imparts a risk for OSCC only when a threshold of >15 units per week is reached, and then the relative risk is modest, around threefold.7 Nonsmokers who use alcohol at this level are more prone to develop cancers of the hypopharynx and the larynx. It has long been recognized that the combined use of alcohol and tobacco in the same individual conveys a multiplied or synergistic degree of risk for OSCC.8 The American Cancer Society reports that cigarette smoking among adults in the United States declined 50% from 1965 to 2005 (from 42% of the population to 21%) but an estimated 45 million Americans continue to smoke. The gender gap narrowed in the 1980s, and now there is a 4% difference in smoking prevelance between White men and women and a 9 % difference between African American men and women. Smoking among teens has declined in the first half of the current decade, estimated at 23% in 2005.9 The risk of cancer due to tobacco exposure is thought to decline with time. However, as there are more ex-smokers in recent years, it may be discovered that the appearance of cancers in this segment of the population has been underestimated previously. Elderly patients who have quit smoking decades earlier can develop cancer of the maxillary alveolus or buccal mucosa.10
The association of the use of smokeless tobacco with oral cancer is less well substantiated than that of cigarettes and alcohol use. Smokeless tobacco use causes visible changes in the oral mucosa including wrinkling with slight elevation and, in some cases, leukoplakia. However, when these areas are biopsied, histologic findings of dysplasia is unusual and somewhat more commonly seen among snuff users than with chewing tobacco use.11 The risk of developing cancer appears to be even more remote. The use of snuff and chewing tobacco has been shown to produce alterations in cell proliferation, apoptosis, and activation of inflammatory elements which, in turn, may be linked to cancer development.12 Typical individuals who develop oral cancer after
smokeless tobacco do so after many decades of use, as late as their eighth decade of life.13
smokeless tobacco do so after many decades of use, as late as their eighth decade of life.13
Oral submucous fibrosis in the setting of areca nut use appears to be a special case of premalignancy associated with oral cancer development. Clinically, this condition is manifested by palpable sheets of fibrous tissue in buccal and labial regions with loss of elasticity and accompanying trismus. Histologically, submucous fibrosis is diagnosed when dense, avascular collagenous connective tissue is present in affected areas together with chronic inflammation and epithelial atrophy.14 In India, oral submucous fibrosis occurs in men five times more often than in women and affects predominantly members of lower socioeconomic classes. The use of areca nut is often combined with other potential carcinogens including snuff, alcohol, and cigarette smoking. The incidence of submucous fibrosis progressing to cancer is estimated between 2% and 7%.15,16 The use of alcohol and tobacco-free betel quid accounts for an increase in cumulative risk of oral cavity cancer of >60 % resulting in an earlier age of diagnosis than seen in nonusers.17 Individuals of South Asian ethnicity are increasingly bringing betel-quid and areca nut use to other regions worldwide.
OSCC in the Absence of Known Carcinogen Exposure
Americans with OSCC that have not used tobacco or alcohol (nonsmokers/nondrinkers [NS/ND]) are disproportionately women (>70% in some series). This group is also more likely to present with early-stage disease. Lateral tongue, buccal, and maxillary alveolar sites are typical.10,18 These patients are quite concerned about why they have oral cancer, and no certain answers exist. Often, the individual patient will have a theory; dental irritation, mouthwash use, cinnamon gum use, and dietary, occupational, and environmental factors have all been suggested. Solid epidemiologic studies of this phenomenon are few, hampered by the small numbers of individuals involved. Still, the appearance of lateral tongue cancer in nonsmokers may be on the increase and constitutes a potentially very fruitful field of inquiry. Myers and others reviewed the M.D. Anderson Cancer Center experience over a 22-year period and found that the percentage of patients with tongue cancer who were <40 years of age increased from 4% in 1971 to 18% in 1993.19 Using the SEER database, an increase of 111% of oral tongue cancer among young white women has been reported between 1975 and 2007 whereas the incidence in all other sites and subpopulations was decreasing.20 It is intriguing that there is such site specificity in this population, predominantly affecting the lateral oral tongue on one side only. Tumors arising on the lateral tongue in NS/ND are sometimes associated with persistent hyperkeratosis to mild to moderate dysplasia in a field around the original tumor site after treatment. In these cases, return of malignancy can occur even after many years of quiescence. Tenderness to certain foods along with increased thickness and friability of lesions are bellwethers of malignant progression. In other cases, simple excision of early tongue SCC results in a nearly undetectable linear scar with no mucosal indication of persistent atypia. In these cases, disease control may be life-long.
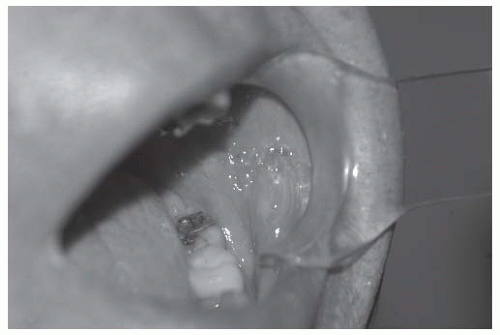
Lichen planus (LP) is a chronic inflammatory disease that preferentially affects the buccal and lateral tongue mucosa and is seen in 1 % to 2 % of the adult population. It presents as a superficial, lacy leukoplakia that fluctuates in intensity in affected individuals over time. Anecdotal observation indicates that OSCC can arise within the field of LP in isolated cases. In these cases, the shallow erosive region occasionally seen in LP may develop a thickened and friable central area of red ulceration indicative of the neovascularity of malignant transformation (Fig. 16-1). The rate of malignant transformation in LP is judged to be quite low (<2%).21
Following the precedent of LP, an autoimmune cause has been widely postulated for the broader phenomenon of SCC in NS/NDs. Chronic inflammation causes a high rate of cell turnover and proliferation in an environment of altered cytokines and reactive oxygen species all of which may contribute to cancer initiation.22 Alternatively, there may be some undiscovered genetic predisposition to oral tongue cancer. One example is Fanconi anemia, a rare inherited genetic condition that leads to early hematologic malignancy. Survivors of this childhood leukemia have a high risk for oral tongue cancer that also arises at a young age.23
OSCC is also seen in other persons who are immunosuppressed after bone marrow or other organ transplant attributed to graft versus host disease (GVHD). These cancers often arise on the alveolus, the buccal, the FOM, or the tongue mucosa.24,25 GVHD is a severe complication of allogenic bone marrow transplantation manifesting chronic inflammation of the oral mucosa as well as the skin, the GI tract, and the liver. Whole-body irradiation for the initial malignancy may increase the risk of secondary cancer. Most second cancers following bone marrow transplantation are hematologic, although SCC is the most common form of solid tumor seen in these individuals.24 OSCC in the setting of GVHD is not limited to the lateral tongue but can affect the dorsal tongue mucosa, the alveolar ridge, and other regions that have been affected by GVHD. These individuals are often much younger than typical OSCC patients, with lesions arising 2 to 10 years after GVHD.
Finally, there may be an undiscovered viral cause of lateral tongue cancer in NS/NDs. Although HPV is widely accepted as a causal factor for cancer arising in the lymphoepithelium of the oropharynx, its presence in lesions of the oral cavity is less common and its contribution to cancer development is controversial. When rigorous detection methods such as in situ hybridization (ISH) are used for HPV assessment, the rate of HPV positivity in oral cancers is small.26 In fact, some tumors that originate in the palatine or lingual tonsil may be mislabeled as oral cavity tumors due to spread from the adjacent epicenter which may have been previously altered by diagnostic biopsy/tonsillectomy. More promiscuous detection techniques based on polymerase chain reaction (PCR) technology may overcall HPV positivity. When detected in very small amount, HPV, although present, may not play a biologically important role in tumorigenesis, bringing into question the relevance of its detection.27 Investigators at University of North Carolina have found that 11/25 lateral tongue cancers from young patients (aged 18-39 years) had overexpression of p16(INK4a) but not HPV-16 by in situ hybridization. In this small series, p16 expression was associated with
improved survival (hazard ratio [HR] = 0.23, p = 0.01).28 In a similar study, none of 32 mobile tongue cancer specimens was found to contain high-risk HPV DNA as assessed by in situ hybridization, although six cases had p16(INK4a) overexpression.29 Risk factors for palate SCC include tobacco and alcohol use, including reverse chutta (cigar) smoking, although denture trauma and poor oral hygiene, as well as a history of syphilis or vitamin deficiency, are sometimes invoked.30
improved survival (hazard ratio [HR] = 0.23, p = 0.01).28 In a similar study, none of 32 mobile tongue cancer specimens was found to contain high-risk HPV DNA as assessed by in situ hybridization, although six cases had p16(INK4a) overexpression.29 Risk factors for palate SCC include tobacco and alcohol use, including reverse chutta (cigar) smoking, although denture trauma and poor oral hygiene, as well as a history of syphilis or vitamin deficiency, are sometimes invoked.30
Oral Cancer Screening. Over the last decade, there has been increasing attention given to the potential community health benefit of oral cancer screening. Both otolaryngologists and dental health professionals have promoted oral screening as a means to improve outcomes of oral cancer care through identification of lesions at the earliest stages. Screening pertains primarily to the oral cavity because of the accessibility of those mucosal surfaces to simple visual inspection. Evaluation elsewhere in the upper aerodigestive tract is more challenging and requires special expertise and equipment.
The Yul Brynner Society, now the Head and Neck Cancer Alliance (http://www.headandneck.org), sponsors an annual national oral cancer awareness campaign that has continued with oral cancer awareness week held each April since 1998. The public health efficacy of oral cancer screening has yet to be demonstrated. One question is who should undergo oral cancer screening. Oral cancer is relatively uncommon, and while there are well-known risk factors for the development of oral malignancy, individuals at risk are often less conscientious about maintaining good health practices and may not avail themselves of screening opportunities.31 Finally, because of the rarity of oral cancers, few primary care dentists and physicians have seen enough oral cancer lesions to develop familiarity and expertise in early detection.
A variety of technological tools have been promoted to assist in oral screening. These tools have in common efforts to enhance visual or spectroscopic features of altered surface epithelium in cases of premalignant or malignant change. One such device, the VELscope, uses autofluorescence technology to examine the oral mucosa. A published study comparing the rate of identification of oral lesions using white light alone versus white light and autofluorescence showed a greater sensitivity for identification of premalignancy on biopsy using VELscope (100% vs. 17%) but also showed a lower specificity for VELscope (74% vs. 97%).32 However, another study failed to show an advantage to autofluorescence over white light visualization with only 81% specificity and 50% sensitivity for autofluorescent detection of lesion compared to 98% specificity and 50% sensitivity for white light.33 Narrow-band imaging endoscopy is another new technology that has been investigated for use in oral cancer detection. This method can be used to identify “brownish spots” within an oral cancer, a feature that may be useful for de novo cancer detection.34 Toluidine blue staining has also been proposed as an adjunct to visual inspection for oral cancer detection. In one recent report, the sensitivity and specificity for detection of cancer or dysplasia was 65.5% and 73.3%, respectively, using toluidine blue.35 Some dental providers now charge patients for the added evaluation using assisted devices such as VELscope. In order to address potential clinical and ethical concerns related to this practice, the American Dental Association (ADA) hosted a working group to review the evidence for device efficacy in the literature. The conclusion of the working group was “that there is insufficient evidence to determine if screening alters disease-specific mortality in asymptomatic people seeking dental care.” Furthermore, the group found, “insufficient evidence that commercial devices based on autofluorescence, [or] tissue reflectance enhance visual detection beyond that achieved through a conventional visual and tactile examination.”36 It would appear that there is no substitute for a clinician being aware of the possibility of oral cancer, the degree of risk born by a particular patient, taking the time to look at the oral mucosa with a good light, knowing what makes a lesion suspicious for malignancy, and exercising appropriate vigilance to arrange a biopsy when indicated.
Another technological adjunct to oral cancer screening is the use of cytologic brush “biopsy.” Oral brush biopsy with computer-assisted analysis is commercially available (OpCDx) and has been reported to have a positive predictive value of 84 % and negative predictive value of 98%.37 This may be helpful in deciding which lesions to refer for surgical biopsy. However, such surgical biopsy remains the gold standard for rigorous and accurate diagnostic assessment of the degree of cancer-associated alteration present in visible lesions of the oral mucosa. The utility of brush biopsy may vary with the degree of experience of the clinician; highly experienced observers may rightly discern degree of suspicion based on subtle aspects such as lesion friability, thickness, tenderness, or variegated coloration, which would render the additional benefit of brush cytology less necessary. The ADA notes that “there is insufficient evidence to assess the validity of transepithelial cytology of seemingly innocuous mucosal lesions,” but the method can “identify dyplastic cells in lesions with a high potential for malignancy.”36 Once again, clinical judgment is a key factor in the detection of oral cancer.
Anatomy of the Oral Cavity
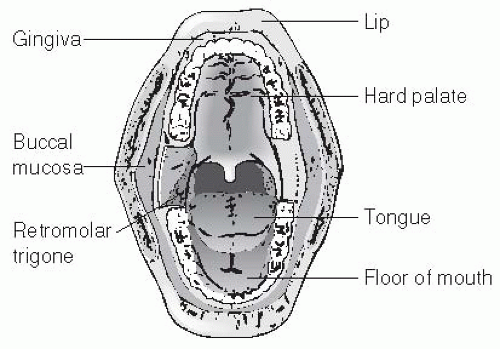
The oral cavity extends from the skin-mucosal junction of the vermillion border of the lips to the oropharyngeal inlet posteriorly. The oropharyngeal-oral junction is bounded superiorly by the hard-soft palate junction, laterally by the anterior tonsillar pillar, and inferiorly by the circumvallate papillae. The lining of the oral cavity is a nonkeratinized stratified squamous epithelial mucous membrane. The submucosa is rich with minor salivary glands, with a high concentration within the hard and soft palates. The oral cavity may be divided into subsites (Fig. 16-2) including the lips, the buccal mucosa, the FOM, the retromolar trigone (RMT), the oral tongue, the hard palate, and the alveolus (maxillary and mandibular). As has been noted, the natural history characteristics of oral carcinoma vary greatly depending on the subsite in which the primary lesion is located. We will therefore consider each of these subsites separately when discussing both anatomy and the management of oral carcinoma.
Lips. The vermilion zone includes the red lip which extends from the fixed gingiva of the labiogingival sulcus up to the vermilion border. The surface landmarks of the lips include the labial commisure laterally, philtrum (Cupid’s bow), and labial tubercle just inferior to the philtrum (upper lip).
Buccal Mucosa. The buccal mucosa is a smooth surface epithelium extending from the oral commissure anteriorly to the
retromolar gingiva posteriorly. The mucosa terminates at the gingivobuccal sulcus of both the maxillary and mandibular gingiva. Its sole landmark is the papule of Stenson’s duct opposite the second maxillary molar. The buccinator muscle lies just deep to the thin mucosa with the buccal fat pad just beyond. The mucosa may be stippled with submucosal minor salivary glands manifest by cream or white colored small, slightly raised spots of approximately 2 mm diameter, called Fordyce spots.
retromolar gingiva posteriorly. The mucosa terminates at the gingivobuccal sulcus of both the maxillary and mandibular gingiva. Its sole landmark is the papule of Stenson’s duct opposite the second maxillary molar. The buccinator muscle lies just deep to the thin mucosa with the buccal fat pad just beyond. The mucosa may be stippled with submucosal minor salivary glands manifest by cream or white colored small, slightly raised spots of approximately 2 mm diameter, called Fordyce spots.
FOM. Tissues of the FOM occupy a semilunar space bounded by the inner aspect of the lower alveolar ridge and the undersurface of the tongue. The transition from FOM to ventral tongue is continuous and indistinct. The posterior extent reaches the base of the anterior tonsillar pillar. The FOM is supported by a muscular sling composed of the mylohyoid, the geniohyoid, and the genioglossus muscles. The sling supports the submucosal sublingual glands, the Wharton ducts, and the minor salivary glands. The lingual and the hypoglossal nerves traverse the submucosal space of the FOM. Surface anatomy includes the lingual frenulum, the Wharton duct papillae, and the sublingual salivary ridge. Prominent veins often are visible below the mucosa as well.
RMT. The RMT is bounded posteromedially by the anterior tonsillar pillar, laterally by the buccal mucosa, and superiorly by the maxillary tubercle. It consists of two surfaces, one facing anteriorly just behind the third mandibular molar and the other facing medially just over the ascending ramus of the mandible. Submucosa here is very thin with bone near the surface.
Oral Tongue. The oral tongue comprises the anterior two thirds lying anterior to the line of circumvallate papillae. The dorsum of the tongue is covered with a thick epithelium interspersed with fungiform, filiform, and circumvallate papillae. Because of the thickness of this mucosa, it may have a wide variety of color which may change in a single individual over time. The thickness also may account for the paucity of neoplasms that arise on the tongue dorsum. The transition to the thin mucosa of the lateral tongue is relatively abrupt. However, in the posterior corner of the oral tongue, the fungiform papillae form vertical ridges which are more red in color than the lateral mucosa more anteriorly. Tongue mucosa lies directly over tongue musculature without intervening fat or salivary gland tissue. The intrinsic tongue musculature (the superior longitudinal muscle, the inferior longitudinal muscle, the transverse muscle, and the vertical muscle) make up the bulk of the freely mobile portion whereas the extrinsic tongue musculature, including the genioglossus, the hyoglossus, the styloglossus, and the palatoglossus comprise its root. The intrinsic muscles are responsible for the remarkable dexterity of the tongue, important in fine motions of articulation and bolus preparation while the extrinsic muscles elevate, depress, protrude, and retract the tongue. The muscles and their neurovascular bundles are bisected by a median raphe made of a fatty-fibrous material. The undersurface (ventral) of the tongue is lined by thin mucous membrane. The lingual frenulum serves as ventral attachment to the FOM. A deep lingual vein is often visible through the mucosa lateral to the lingual frenulum.
Hard Palate. The hard palate is a semilunar area bounded by the upper alveolar ridge anteriorly and laterally and the soft palate junction posteriorly. The submucosa here is tough and thick. A bony torus palatine may provide surface contour to an otherwise regular concavity. The greater palatine foramen is located in the palate bone near the second molar tooth and contains the descending palatine artery on each side that services the palate mucosa.
Alveolar ridge. Bony structures supporting the teeth and periodontal tissue arise from the FOM and descend from the hard palate. Here the mucosa is thin and tightly adherent to the periosteum. When teeth have been extracted, the bony elevation is often partially lost, particularly on the lower ridge. Mucosa here envelopes the neck of teeth with a small periodontal sulcus. Dental hygiene affects the quality of this mucosa with common inflammation and retraction of soft tissue from the teeth when hygiene is insufficient, and gingival hypertrophy in the setting of certain diseases and drug therapy.
The blood supply of the oral cavity is derived from branches of the paired external carotid arteries. The arterial supply of the lips is the labial arteries, which are branches of the facial artery. These paired arteries anastomose in the midline. The buccal mucosa likewise is supplied from branches of the facial artery. The lingual artery supplies the tongue, coursing deep in the extrinsic musculature below the digastric tendon and the posterior border of the hyoglossus muscle. The anterior tongue derives all of its blood supply from the lingual artery, with little contribution from the FOM mucosal vessels. The dominant blood supply of the hard palate is via the greater palatine artery and the superior alveolar arteries. Branches of the internal maxillary artery and inferior alveolar artery supply the maxillary and mandibular alveolar ridge. Branches of the facial artery supply the submandibular gland, the sublingual gland, and some of the FOM mucosa.
The oral cavity has a complex system of lymphatic drainage, supplying the first echelon basins of the submental, the submandibular, and the upper jugular systems and has recently been the subject of a review.38 A more detailed analysis of lymphatic drainage patterns is included in the management section for each subsite.
Innervation of the oral cavity can be classified as sensory, special sense, and motor. The trigeminal nerve is the primary sensory supply of the oral cavity through its maxillary and mandibular branches. The lingual nerve (V3) supplies the sensory distribution to the anterior two thirds of the tongue and adjacent FOM. Taste fibers course with the lingual nerve after originating in the geniculate ganglion and traversing in the chorda tympani, then joining the lingual nerve. Motor control of the tongue is supplied by the hypoglossal nerve. Sensation of the lower lip and gum is provided by the mental nerve, a branch of V3. The hard palate, upper gum, and upper lip are innervated by the palatine and the labial branches of V2. The muscles of mastication are controlled by the second and third divisions of the trigeminal nerve whereas the lip and cheeks are innervated by the facial nerve.
Natural History of Oral Cancer (Pathology, Pathogenesis, and Patterns of Spread)
Molecular Underpinnings of Oral Cancer. Over the past two decades, a great deal of insight has been gained into the molecular processes that lead to the development and behavior of malignant neoplasms, including cancer of the oral cavity. It is increasingly recognized that tumor-specific genetic and epigenetic alterations affect key physiologic pathways that control functions such as cell proliferation, DNA repair, neoangiogenesis, motility, and metastasis. Genetic alterations include mutation, chromosomal rearrangement, and loss or amplification of segments of DNA while epigenetic alterations, particularly promoter hypermethylation, affect gene function through alteration of transcription. The effect of specific molecular alterations interweave in a complex manner such that a single event or intervention may have widely variable phenotypic result. Point mutation of the p53 gene occurs in more than one half of all OSCC,10 eliminating or reducing important cellular activities affecting cell cycle control and programmed cell death (apoptosis) in response to genotoxic stress. However, this genetic alteration alone is insufficient to account for the cancer
phenotype, and the discovery of other genes with a substantial rate of mutation in HNSCC has not occurred. New data are emerging indicating that not all p53 mutations have the same effect, with those that disrupt the DNA-binding domain three-dimensional configuration being associated with poorer clinical outcome.39 Another tumor-specific target commonly affected in HNSCC is the Rb pathway which regulates the cell cycle. Loss of function of Rb and other members of this pathway including p16 results in deregulation of cell growth and tumorigenesis. Mutations of p16 are rare in HNSCC, but homozygous deletion occurs in >50 % of cases. In another substantial group of cases, p16 transcription is blocked by hypermethylation of the promoter region.40
phenotype, and the discovery of other genes with a substantial rate of mutation in HNSCC has not occurred. New data are emerging indicating that not all p53 mutations have the same effect, with those that disrupt the DNA-binding domain three-dimensional configuration being associated with poorer clinical outcome.39 Another tumor-specific target commonly affected in HNSCC is the Rb pathway which regulates the cell cycle. Loss of function of Rb and other members of this pathway including p16 results in deregulation of cell growth and tumorigenesis. Mutations of p16 are rare in HNSCC, but homozygous deletion occurs in >50 % of cases. In another substantial group of cases, p16 transcription is blocked by hypermethylation of the promoter region.40
Promoter hypermethylation is one form of epigenetic control of gene expression. Many regions of tumor-specific promoter hypermethylation have been described in HNSCC, accounting for a lack of expression of other genes with key tumor suppressor functions.41 One distinct example is the Deleted in Colon Carcinoma (DCC) gene, frequently mutated in colon cancer, but rarely in HNSCC. Promoter hypermethylation of Deleted in Colon Carcinoma has been shown to be a frequent event in HNSCC.42 Other key genes so altered included MDM2, Interferon-alpha, and RAR-β. MicroRNA, short 22-base sequences that can selectively block gene transcription, promises to provide yet another substantial mechanism for epigenetic tumor-specific alteration.
Studies of the rate and type of genetic alterations in oral premalignant lesions have helped sketch a tumor progression pathway for oral mucosal malignancy. By examining a spectrum of histologic lesions from atypia to dysplasia to carcinoma in situ to invasive cancer, and cataloging the molecular events present, a general pattern of accumulated alterations correlated with progressive phenotypic change has been established.43 Some genetic alterations such as loss of p16 expression, occur with high frequency in very early lesions such as atypia and dysplasia and do not increase greatly with further histologic progression. Other alterations only appear with substantial frequency in invasive SCC. The spectrum of genetic alterations in oral premalignant lesions may serve a prognostic role, providing evidence as to the risk of progression to invasion. Several studies have shown that loss of heterozygosity (LOH) of key gene regions including 3p, 9p, and 17p portend a significantly increased rate of progression.44,45
Patterns of Spread. Malignant lesions of the oral mucosa arise from a region populated by clonally altered cells, a phenomenon known as field cancerization.46 The involved field may be clinically apparent with regions of leukoplakia and erythroplakia, but more often it is unapparent and undetectable without sampling and sophisticated molecular methods. Enhanced visualization may be provided by supravital staining with tolonium chloride, or with measures of reflectance or absorbance of specific wave-lengths of light.47,48 The effect of field cancerization is to spawn neighboring lesions either contemporaneous to an index lesion or some time later (field recurrence). The ventral tongue and FOM are common locations for this phenomenon, presumably due to common exposure to carcinogens.
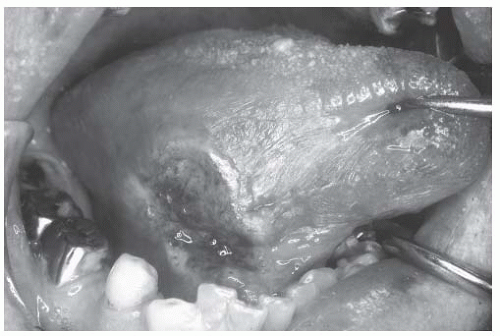
Tumor Histology. By far, the majority of oral cavity cancers are OSCC. SCC arises from a series of genetic and epigenetic alterations that abrogate the function of key tumor suppressor genes at the crossroads of functional pathways.43 The typical progression from cytologic atypia and hyperplasia to various degrees of dysplasia to in situ carcinoma and then invasive carcinoma may be manifest as erythroplakia, leukoplakia, or be largely unapparent. Leukoplakia, or white patch, may occur in the absence of malignant progression in the setting of local trauma or irritation. Palpable thickness, firmness, and friability (ready blushing or bleeding with manipulation) are telltale features of malignant transformation.
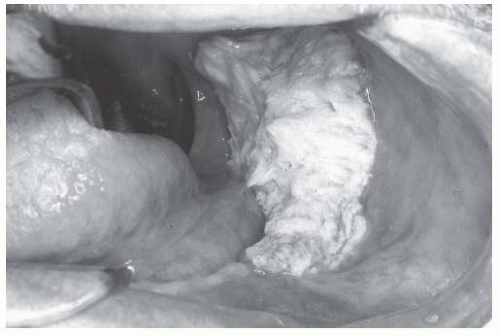
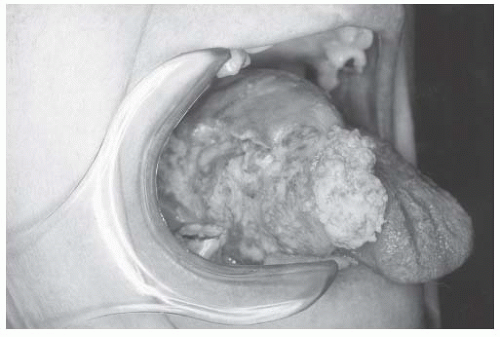
There are distinct patterns of tumor growth that characterize subclasses of OSCC. Verrucous change is one such variant. This entity is prone to recur after excision and sometimes spreads across mucosal surfaces to involve large portions of the oral cavity. White, patchy, thickened change of the mucosa characterizes early verrucous leukoplakia. More advanced verrucous change becomes velvety and exophytic (Figs. 16-3 and 16-4). Histologically, these lesions exhibit bland cytologic change with broad pushing borders of the invasive component. This feature, together with the thick nature of advanced lesions, makes the diagnostic distinction between invasive and preinvasive verrucoid lesions difficult, with many biopsies failing to obtain full thickness of tissue that adequately demonstrates the infiltrative interface.49
The anatomic features of different regions within the mouth affect the pattern of tumor growth and spread. The dorsum of the tongue and the hard palate with their thick mucosa and submucosa are somewhat impervious to the development of OSCC. These regions are also located above pooled secretions which may reduce their exposure to carcinogens. Once cancer invades the intrinsic musculature of the tongue, there is no substantial barrier to its further spread between muscle fascicles. This fact makes delineation of the necessary deep boundary of resection difficult.
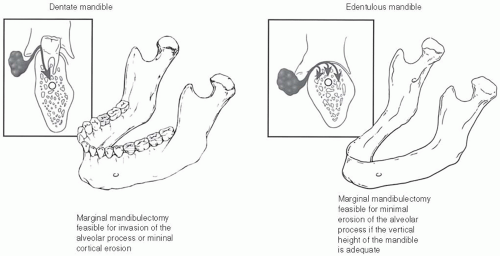
Detection of invasion of tumor into the mandible is important for staging and treatment planning. While cortical bone provides a substantial barrier to tumor, the lack of cortex in an empty tooth socket permits entry of invasive cancer into the marrow
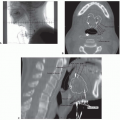
space and mental nerve canal. Once this has occurred, tumor can spread laterally and medially to the extent of the marrow space without obvious external evidence (Fig. 16-5).50 Computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) scanning all can help detect the presence and degree of bone invasion (Fig. 16-6).51
space and mental nerve canal. Once this has occurred, tumor can spread laterally and medially to the extent of the marrow space without obvious external evidence (Fig. 16-5).50 Computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) scanning all can help detect the presence and degree of bone invasion (Fig. 16-6).51
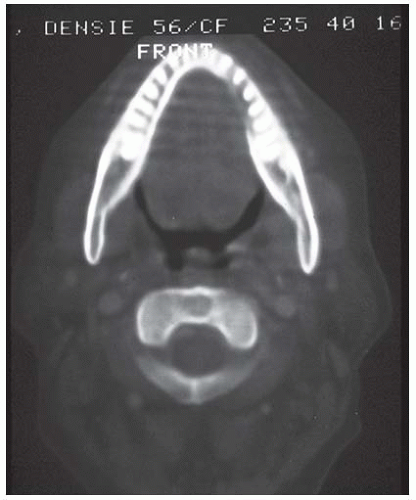 FIGURE 16-6. CT scan with bone windowing shows erosion of the inner cortex of the left mandible, lingual surface due to an adjacent floor of mouth tumor. |
Nonsquamous Oral Cancers. In addition to SCC, the oral cavity may develop minor salivary glandular carcinomas and a variety of sarcomas. Minor salivary gland tumors are malignant in the majority of cases. Specific malignant lesions include mucoepidermoid carcinoma, polymorphous low-grade adenocarcinoma, adenoid cystic, acinic cell, clear cell, and myoepithelial carcinoma, to name several. The most common location for all tumors in that series was the palate, accounting for 57% (89/156) of cases including most of the mucoepidermoid carcinomas, polymorphous low-grade adenocarcinomas, and adenoid cystic carcinomas.52 Some of these entities have distinctive clinical presentations, such as polymorphous low-grade adenocarcinoma, which along with pleomorphic adenoma tend to arise laterally at the hard palate-soft palate junction. In a report from a single institution spanning 20 years of case accumulation, mucoepidermoid carcinoma was the most common histologic category among minor salivary gland malignancies of the oral cavity, accounting for 22% of 156 collected cases.
A wide variety of sarcomas arise within the oral cavity, although all are rare. Kaposi sarcoma is a distinctive entity typically affecting individuals with untreated human immunodeficiency virus (HIV) disease. It begins as an inflammatory, hyperplastic, angiogenic lesion. The gingiva, the palate, and the tongue are most commonly affected. HAART has reduced the rate of Kaposi sarcoma by up to 50%.53 Osteosarcoma of the mandible and maxilla is the most common sarcoma of the oral cavity, presenting as a painless mass or accompanied by dental pain and loose teeth.54 Other sarcomas that may appear in the oral cavity include chondrosarcoma, leiomyosarcoma, alveolar soft part sarcoma, fibrosarcoma, and liposarcoma.55
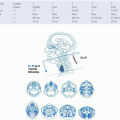
Lymphatic Metastasis from Oral Cancer
Lymphatic extension from the oral cavity follows well-described patterns (Fig. 16-7). Level I nodes are the primary echelon basin for spread from the anterior FOM and the tongue tip, central lips, and mesial alveoloar surfaces of the maxilla and the mandible. Level II nodes drain the lateral FOM and the tongue. Several
series have documented the relationship between tumor thickness of cancers of the FOM with the likelihood cervical metastasis, with tumor thickness >1.5 mm associated with an increased risk of lymph node metastasis.56,57 Likewise, for tumors of the FOM or the oral tongue, Spiro and others, in a review of 105 patients staged NO, reported a 12% rate of regional failure with a primary tumor thickness <2 mm as opposed to a failure rate of 47% for tumors thicker than 2 mm. Clearly, those patients with clinically NO necks with a tumor thickness >1.5 to 2 mm warrant consideration for proactive management of the neck.58 Whereas tumor thickness has heretofore been determined by microscopic evaluation of a full-thickness tumor biopsy, intraoral ultrasound has been explored as a noninvasive alternative.59
series have documented the relationship between tumor thickness of cancers of the FOM with the likelihood cervical metastasis, with tumor thickness >1.5 mm associated with an increased risk of lymph node metastasis.56,57 Likewise, for tumors of the FOM or the oral tongue, Spiro and others, in a review of 105 patients staged NO, reported a 12% rate of regional failure with a primary tumor thickness <2 mm as opposed to a failure rate of 47% for tumors thicker than 2 mm. Clearly, those patients with clinically NO necks with a tumor thickness >1.5 to 2 mm warrant consideration for proactive management of the neck.58 Whereas tumor thickness has heretofore been determined by microscopic evaluation of a full-thickness tumor biopsy, intraoral ultrasound has been explored as a noninvasive alternative.59
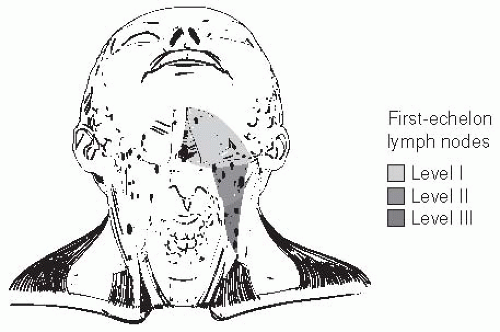 FIGURE 16-7. First-echelon lymph nodes at risk for metastasis from carcinoma of the oral cavity are located in the supraomohyoid triangle. |
Distant dissemination of OSCC is uncommon, particularly at the time of initial presentation. Spread to liver, lung, and bones does occur, more commonly in advanced cases, particularly in the setting of extensive nodal metastasis.
Clinical Presentation. Oral malignancies typically present as visible lesions ranging from deep ulceration to fungating exophytic growth. They may be white or red compared to normal mucosa (Fig. 16-8). Cancers tend to be firm and thick compared to normal surrounding tissue when palpated. Malignant lesions may be painless, but more commonly are the source of deep persistent pain which may be referred to the ipsilateral ear. Bleeding is a common feature, attributed to neovascularity of malignant angiogenesis and fragility of feeding vessels. Loosened teeth or poorly fitting dentures may draw attention to alveolar ridge lesions. In some cases, a firm submandibular or upper jugular lymph node may be the presenting sign of OSCC.
Diagnostic Evaluation and Clinical Staging
A thorough history must be obtained to initiate the workup of an oral cancer, ascertaining the time course of appearance and progression of the lesion, exposure to known risk factors and pertinent medical history. A complete head and neck exam follows with attention to the physical features of the lesion (precise location, dimension, thickness, friability) as well as screening for possible second lesions. Visualization requires good illumination, gauze, and instruments to move the lips and the tongue in order to see all surfaces thoroughly. Palpation is of great importance to detect submucosal extension and tumor thickness. Careful attention to pertinent lymph node basins in the neck follows. Bimanual palpation of the submental and submandibular regions is quite helpful to discern the presence of small but firm nodes in this region. Posteriorly positioned lesions of RMT, posterolateral tongue, or posterior FOM, because of their less accessible location in the oral cavity, are sometimes difficult to assess (Fig. 16-9). Sensory nerve distribution here is less precise making it difficult for some patients to accurately identify the source of cancer pain. Gag reflex and tongue resistance to manipulation further hinders efforts by the clinician to locate visible posterior tumor or precursor lesions on oral screening evaluations. These tumors will often remain unnoticed until the patient presents with progressive persistent pain, dysphagia and weight loss, referred otalgia, or trismus.
Assessing mandibular invasion by physical exam alone can be challenging. Involvement of the maxilla may also occur, and masticator space involvement is a particularly poor prognostic factor.60 Physical mobility of the tumor mass relative to the mandible can be judged by bimanual manipulation which may be facilitated by general anesthesia in some cases. Imaging by CT may help to clarify the status of bony involvement. However, the sensitivity of CT for determining bony involvement is only 50%. Lane and others, in a review of patients treated for cancer of the RMT over a 14-year period, found that bony invasion was not
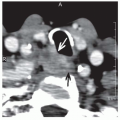
identified correctly by radiograph in 27% of patients with preoperative CT scans.61 The reported accuracy of MRI in detecting mandibular invasion in the literature has also been mixed.62,63,64 PET scanning may help with detection of invasion in the setting of intact dentition, whereas MRI appears to be superior for detection of marrow involvement (Fig. 16-10).51
identified correctly by radiograph in 27% of patients with preoperative CT scans.61 The reported accuracy of MRI in detecting mandibular invasion in the literature has also been mixed.62,63,64 PET scanning may help with detection of invasion in the setting of intact dentition, whereas MRI appears to be superior for detection of marrow involvement (Fig. 16-10).51
Radiologic Evaluation. The timing and type of radiographic imaging selected for workup of oral cancer depends on several issues. Small surface lesions may not require any imaging since they will not be visible and the risk of nodal spread is small. CT, MRI, and PET scanning can help delineate the extent of larger lesions, particularly pertaining to deep tongue and mandibular invasion as well as nodal metastasis. The timing of PET scanning is particularly worthy of comment since recent biopsy may result in inflammatory metabolic activity in regional nodes rendering a false-positive uptake that could be interpreted as indicative of metastatic tumor. CT with intravenous contrast is often the first choice in this setting, as it provides excellent visualization of bone/soft tissue borders, particularly important in oral cavity lesions. Contrast enhancement is present in many tumors due to increased blood supply. MRI is the imaging modality of choice when evaluating soft tissue lesions and may be more useful for demonstrating the extent of the primary lesion. In particular, MRI provides superior imaging of lesions of the oral tongue and the tongue base. For lesions where there is a question of base of skull involvement, MRI likewise provides superior imaging.
PET with fluorodeoxyglucose (FDG) is now a standard component of the staging of head and neck cancers and in screening for second primary cancers and regional and distant metastatic disease. However, the contribution of PET imaging to the preoperative workup for staging the clinically NO neck remains uncertain and may not be a replacement for staging neck dissection.65,66 Schwartz and others, in a prospective series comparing pretreatment CT alone with fusion PET-CT in patients with head and neck cancer, found that PET-CT was superior in identifying regional lymph node metastasis. Although the majority of these patients had advanced-stage disease (stage III or IV), their findings provide evidence to support including functional PET imaging in the preoperative workup.67 PET/CT scanning promises increased sensitivity for detection of distant metastatic disease over that provided by conventional CT scanning.68 As the rate of synchronous primary cancers may range from 2.6% to 15%, a systemic workup is of importance preoperatively, particularly for patients who have been smokers. Again, the relative value of PET/CT compared to panendoscopy for detection of second primary cancers of the upper aerodigestive tract is yet to be determined.69,70
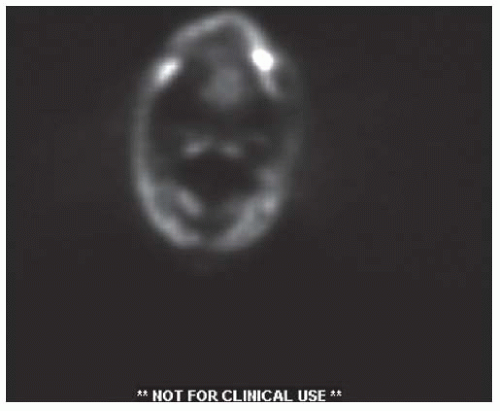 FIGURE 16-10. FDG-PET scan showing high metabolic uptake in the marrow space of the left hemimandible. |
Biopsy. An incisional biopsy to confirm the diagnosis of a suspicious lesion is mandatory. It may be necessary to sample several areas if there are regions with varying appearance. Because of the highly specialized histologic criteria for classification of the stages of oral mucosal premalignancy and the spectrum of minor salivary gland tumors, the experience of the pathologist reading the biopsy material is a key factor in proper workup. Biopsy of most sites within the oral cavity is feasible in the office under local anesthesia when the patient has even modest ability to cooperate.
Tumor Staging. The stage of oral cavity cancers is based on the greatest dimension of the surface extent. A tumor may be staged T4 due to deep tongue, soft tissue, or bone invasion regardless of surface dimension (Table 16.2). Staging for nodal metastasis is identical to that of other head and neck sites. T and N stage are both excellent prognostic factors in most clinical series with good delineation between stage categories.3,71 However, the UICC sixth edition staging categories of IVa versus IVb and T4a versus T4b did not differentiate between statistically different subpopulations in a recent report of 300 OSCC patients indicating a weakness in distinguishing between low- and high-risk advanced tumors using this schema.
MANAGEMENT STRATEGIES
Pretreatment Considerations
Several factors must be taken into consideration when choosing a management strategy for the patient with oral cancer. General physical health and functional status are important in determining the suitability of the patient for both surgical and nonsurgical approaches. This includes evaluating and optimizing comorbidities and the nutritional status of the patient. The patient’s ability
to undergo extensive surgery, cooperate with rehabilitation, and withstand changes in safe and efficient swallowing must be considered. The expectations of the patient for postoperative quality of life (QOL) must also be considered, particularly pertaining to cosmesis, articulation, and the ability to eat a normal diet in social situations. When tumor factors prevent adequate oral intake prior to treatment resulting in weight loss and debility, placement of a gastrostomy tube may be indicated. Several weeks of hyperalimentation are required to reverse starvation and promote anabolic enzymes needed for wound healing. An evaluation of cardiovascular health is also required for any patient undergoing surgery, particularly in cases requiring a more complex reconstruction, such as free tissue transfer. In fact, severe peripheral vascular disease may preclude microvascular reconstruction because of a lack of suitable recipient vessels in the neck.
to undergo extensive surgery, cooperate with rehabilitation, and withstand changes in safe and efficient swallowing must be considered. The expectations of the patient for postoperative quality of life (QOL) must also be considered, particularly pertaining to cosmesis, articulation, and the ability to eat a normal diet in social situations. When tumor factors prevent adequate oral intake prior to treatment resulting in weight loss and debility, placement of a gastrostomy tube may be indicated. Several weeks of hyperalimentation are required to reverse starvation and promote anabolic enzymes needed for wound healing. An evaluation of cardiovascular health is also required for any patient undergoing surgery, particularly in cases requiring a more complex reconstruction, such as free tissue transfer. In fact, severe peripheral vascular disease may preclude microvascular reconstruction because of a lack of suitable recipient vessels in the neck.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree