Diarrheal disease is the second most common cause of death in children younger than 5 years worldwide, according to the World Health Organization (WHO). In the United States, viruses are the major cause of acute gastroenteritis in children; norovirus, rotavirus, adenovirus, astrovirus, and sapovirus are the most commonly detected. Viral gastroenteritis also affects immunocompromised children, in whom it causes more morbidity and mortality, including longer duration of illness, increased hospitalization rates, renal injury, and graft rejection. ,
With increasing availability and use of molecular diagnostic testing, especially multipathogen platforms, etiologic diagnosis of viral gastroenteritis is increasing and improved epidemiologic data are becoming available. No U.S. Food and Drug Administration (FDA)-approved medications exist for the treatment of viral gastroenteritis (except for cytomegalovirus [CMV]. For a complete discussion of CMV disease in immunocompromised children, see Chapter 17 ). Therapeutics for viral gastroenteritis are an area of active research, especially among immunocompromised patients, where disease is more severe and prolonged. Although an effective vaccination against rotavirus is now being used worldwide, vaccines for the other major viral causes of gastroenteritis do not exist. This too is an area of active research.
Norovirus
Norovirus infection is the most common cause of acute gastroenteritis worldwide, accounting for approximately 20% of all cases. Noroviruses are part of the family Caliciviridae and are 27- to 40-nm, non-enveloped, single-stranded ribonucleic acid (RNA) viruses. Owing to genetic drift, the genus norovirus is genetically diverse and divided into seven genogroups, which are further subdivided into more 40 genotypes. Three of the genogroups (GI, GII, and GIV) have been isolated in humans, with GII.4 causing the majority of illness.
Epidemiology and risk factors
Norovirus is transmitted through the fecal-oral route by close person-to-person contact. It can also be transmitted through contaminated food or water, and environmental contamination has been implicated in outbreaks. In addition, because norovirus is present in the vomitus of infected individuals, it can be spread by droplets to those who are in close contact with or caring for individuals who are vomiting. Norovirus is very infectious and a low inoculum is needed to cause disease. Norovirus virions are quite stable in the environment and can remain infectious on fomites for at least 7 days. Norovirus can be detected in the stool of asymptomatic individuals; one meta-analysis found norovirus in the stools of 7% of healthy controls (3% to 10%). This is likely a consequence of asymptomatic infection and/or convalescent excretion after illness has resolved. The role of asymptomatic shedding in transmission is unknown.
Noroviruses are a leading cause of both sporadic cases and outbreaks of acute gastroenteritis in the community worldwide. Community outbreaks occur year-round but the majority occur in the winter. They are also important causes of institutional and nosocomial infection and outbreaks. A recent survey of U.S. hospitals revealed that noroviruses were among the most commonly detected nosocomial pathogens and caused the highest rate of hospital unit closures.
Norovirus affects all age groups. Globally, norovirus prevalence in acute gastroenteritis cases in children younger than 5 years was 18% (15% to 20%) and 19% (7% to 21%) in all age groups. In the United States, norovirus is associated with 19 million to 21 million episodes of gastroenteritis and up to 71,000 hospitalizations annually. Nearly 800 deaths are caused by norovirus each year, which is the second most common cause of gastroenteritis-related death in the United States. Deaths occur disproportionately among individuals with chronic and/or immunocompromising conditions, such as transplantation and/or chemotherapy. Norovirus has been detected in 22% to 32% of immunocompromised children with diarrhea. , ,
Clinical manifestations
Symptoms of norovirus infection are similar to other causes of acute viral gastroenteritis and include vomiting, abdominal pain, watery, nonbloody diarrhea, and low-grade fever after an incubation period of about 24 hours. Vomiting is common and may be the only symptom. Symptoms usually last 12 to 60 hours in immunocompetent individuals. In contrast, the immunocompromised can have more severe and prolonged symptoms. A study of pediatric oncology patients with diarrheal illness found that 44% of these patients with norovirus required hospitalization. The median duration of diarrhea was 6 days (interquartile range 3 to 10 days). Another study of immunocompromised pediatric patients found that the mean duration of symptoms was 10 days; the majority (70%) had symptoms for 4 days or longer and more than 20% had symptoms for longer than 14 days. In addition, nearly half of these children had seven or more diarrheal episodes daily.
Chronic norovirus diarrhea (lasting ≥4 weeks) has been well described, especially in solid organ transplant (SOT) recipients and other immunocompromised individuals. Illness can be associated with significant weight loss, failure to thrive, dehydration requiring hospitalization, renal dysfunction, and relapsing diarrhea. One study found that the strongest association with duration of diarrhea was having received induction immunosuppression with antithymocyte globulin and having received plasmapheresis, in addition to human antigen leukocyte– and/or ABO-incompatible kidney transplant status, suggesting that more significant immunosuppression could be associated with a more severe course. Another study of norovirus in SOT patients also found that CMV infection in the 90 days preceding norovirus diagnosis and nausea at presentation were two significant risk factors for diarrhea persisting more than 2 weeks.
Disease prophylaxis and prevention
No licensed norovirus vaccines exist. Five different norovirus vaccines are currently being developed. Two vaccines have been studied in humans with promising results. One safety and immunogenicity trial in children is ongoing (NCT02153112). All of these are virus-like particle vaccines targeting the major capsid protein. It is unclear whether candidate vaccines would be effective in patients with significant immunosuppression. However, an effective vaccine might still protect immunocompromised patients via herd effects.
Diagnosis
The genetic (and antigenic) diversity of noroviruses has led to low sensitivity for antigen detection tests, whereas polymerase chain reaction (PCR)-based detection has proven very sensitive. Multiple singleplex PCR-based tests that have high sensitivity are commercially available. Recently sensitive multiplex PCR-based tests for multiple gastrointestinal pathogens (including norovirus) have become increasingly available and used. None of the three commercially available multipathogen gastrointestinal panels can distinguish between norovirus GI and GII. Because norovirus can be shed by healthy and/or asymptomatic individuals and given the high sensitivity of many of the PCR-based assays, clinicians must interpret a positive test result in the context of the clinical situation and symptoms.
Treatment
Clinical management of norovirus is supportive and targeted at maintaining adequate hydration, nutrition, and electrolyte balance. The WHO recommends low-osmolarity oral rehydration solution as opposed to traditional oral rehydration solution, and it has been shown to decrease vomiting and stool output in children with acute gastroenteritis. Ondansetron reduces vomiting in children with acute gastroenteritis and decreases need for intravenous hydration.
Multiple potential therapies have been tried, with limited success in immunocompromised patients, to manage the more severe manifestations. Reduction of immunosuppression, when possible, often is helpful in immunocompromised patients with prolonged and/or severe symptoms but may be associated with the risk of graft rejection. One small study in kidney transplant patients described decreased immunosuppression associated with clinical improvement, but continued viral shedding in two-thirds of the patients.
No medications are currently approved for treatment of norovirus infection. Nitazoxanide is an antiparasitic drug licensed in the United States for treatment of Cryptosporidium parvum and Giardia lamblia in adults and children older than 12 months. However, nitazoxanide has been reported to have broad-spectrum antiviral activity as well through a variety of poorly understood mechanisms. A recent systematic review of five studies of nitazoxanide for the treatment of acute gastroenteritis caused by norovirus, rotavirus, or adenovirus showed use of this agent shortened duration of diarrhea by approximately 24 hours compared with placebo. Multiple published case reports describe the use of nitazoxanide for norovirus gastroenteritis in immunocompromised individuals with variable effect on clinical symptoms and/or viral shedding. , A multicenter, randomized, placebo-controlled trial of nitazoxanide for treatment of symptomatic norovirus infection in SOT and hematopoietic stem cell transplantation (HSCT) recipients is ongoing and will hopefully provide definitive evidence regarding the efficacy of this agent for norovirus disease (NCT03395405).
Both intravenous and enteral administration of immunoglobulin have been used in immunocompromised patients with norovirus, but published experience is limited to case reports. Two single case reports in heart and pancreas transplant recipients cited no difference in symptoms after intravenous immunoglobulin administration. Enteral administration of immunoglobulin has been described in case reports as well, with mixed results. Concern exists about protein degradation by the acidic environment, so jejunal administration has been used. A case-control study of pediatric oncology and transplant patients (primarily SOT) evaluated enteral immunoglobulin in 12 patients and found a trend toward resolution of diarrhea and stool output 7 days after treatment. However, the study did not find a difference in length of stay, hospitalization cost, or time to resolution of diarrhea. Because of the possible antiviral effects of mammalian target of rapamycin inhibitors, substituting rapamycin for other immunosuppressants is a theoretical strategy whose use has been reported in a few cases of chronic norovirus. Additional studies of potential therapeutics are clearly needed for immunocompromised pediatric patients.
Infection prevention and anticipatory guidance
Norovirus is relatively resistant to many available disinfectants, including alcohol-based hand sanitizers. It is also relatively resistant to many commonly used hospital disinfectants based on phenolic compounds (triclosan and quaternary ammonium). Bleach-based solutions are recommended for environmental cleaning. Handwashing with soap and water is effective at preventing transmission, including in institutional settings. Guidelines recommend standard precautions for viral gastroenteritis, including norovirus, with the use of contact precautions for diapered or incontinent patients for the duration of illness. Owing to the description of nosocomial outbreaks and the potential for serious consequences among transplant patients, we recommend contact precautions for all transplant patients with viral gastroenteritis, In addition, transmission has been described through aerosolized vomitus or fecal material ; thus droplet precautions are appropriate if a patient is actively vomiting. Ideally, these patients should be in private rooms. Given the infectiousness of symptomatic individuals, infected health care workers should be excluded from work for at least 48 hours after resolution of symptoms.
Rotavirus
Rotavirus is the leading cause of severe gastroenteritis among children worldwide. Despite the success of rotavirus vaccines, more than 90 million infants still lack access to a rotavirus vaccine, and rotavirus infections are still responsible for 180,000 to 450,000 deaths each year in children younger than five years globally. Rotaviruses are non-enveloped, double-shelled RNA viruses in the family Reoviridae. The genome is composed of 11 segments of double-stranded RNA, coding for six structural and five nonstructural proteins. One of these nonstructural proteins, NSP4, is an intracellular receptor and has been shown to have direct toxic effects on the gastrointestinal mucosa.
Epidemiology and risk factors
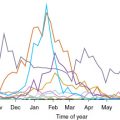
Young children 6 months to 2 years of age who are immunologically naïve are at highest risk for rotavirus infection. Most children have experienced an initial rotavirus infection by age 5. Older children and adults who are immunocompetent are usually asymptomatic or have mild disease with subsequent episodes of infection. Transmission occurs via a fecal-oral route, usually through direct contact between people, and a small inoculum, such as 100 virions per gram of stool can be contagious. Transmission also can occur via ingestion of contaminated water or food and contact with contaminated surfaces or objects. Family outbreaks are common, and up to 50% of exposed immunocompetent children within a household develop rotavirus gastroenteritis. Rotavirus is present in the stools of infected children several days before and after the onset of clinical symptoms. Asymptomatic excretion of rotavirus in stool is relatively common and likely plays a role in transmission. The virus is stable in the environment and can be found on toys or hard surfaces. The incubation period is short, usually less than 2 days. In the United States, incidence peaks during late winter and early spring with annual epidemics occurring from December through June.
Clinical manifestations
Rotavirus infections are characterized by watery nonbloody diarrhea, vomiting, fever, or abdominal pain. Vomiting usually lasts for 2 to 3 days and other symptoms resolve within a week. Gastroenteritis caused by rotavirus cannot be clinically distinguished from that caused by other viral enteric pathogens. Severe cases can result in dehydration with shock, electrolyte imbalance, and death. Central nervous system involvement with seizures and encephalopathy has been described and rotavirus has been detected in cerebrospinal fluid on occasion. Necrotizing enterocolitis, intussusception, biliary atresia, and diabetes mellitus have also been described in association with rotavirus infection. However, it is uncertain whether rotavirus is an etiologic factor for these clinical syndromes.
Among immunocompromised children, particularly those with T-cell immunodeficiency or those who have undergone HSCT, rotavirus infections can cause severe disease with prolonged diarrhea fever, dehydration, electrolyte imbalances, acidosis, and mortality. Among 183 pediatric patients who received an allogeneic HSCT at St. Jude Children’s Research Hospital, 36 (19.7%) had at least one episode of rotavirus infection over the first 3 years after transplant and the median duration of diarrhea was 17.5 days (range 4 to 122 days). In a retrospective case-control study among pediatric oncology patients, the median duration of rotavirus-related symptoms was 7 days (range 4 to 34 days) and the median duration of viral shedding was 17 days (range 4 to 73 days). Children with SOT usually have more severe disease compared with healthy children and may experience prolonged hospitalization; however, the infection usually resolves without treatment. In addition, rotavirus may promote acute cellular rejection, particularly among intestinal transplant patients.
In addition, rotavirus infection can lead to increased serum levels of particular immunosuppressive agents in organ transplant recipients, particularly in liver transplant recipients. Fruhwirth and colleagues described three pediatric SOT recipients in whom rotavirus infection caused increased trough levels of tacrolimus. Although exact mechanisms are uncertain, as tacrolimus is significantly metabolized in the intestine, increased intestinal permeability and decreased gastrointestinal transit time, which would enhance drug availability in areas with lower intestinal metabolism such as the colon, have been proposed as potential mechanisms for increasing trough levels of tacrolimus.
Disease prophylaxis and prevention:
The most effective way to prevent rotavirus infections is through the use of the rotavirus vaccine. The WHO, the American Academy of Pediatrics, and other institutions recommend universal infant immunization against rotavirus. Two live attenuated oral rotavirus vaccines are licensed for use in the United States: pentavalent human-bovine rotavirus reassortant vaccine (RV5, PRV, RotaTeq [Merck, Kenilworth, NJ]) and attenuated human rotavirus vaccine (RV1, HRV, Rotarix [GlaxoSmithKline Biologicals, Rixensart, Belgium). RV5 is administered in three oral doses at 2, 4, and 6 months of age, whereas RV1 is administered in two oral doses at 2 and 4 months. Both vaccines may be started as early as 6 weeks of age and the first dose of both vaccines should be given before 14 weeks 6 days of age. No rotavirus vaccine should be administered to infants older than 8 months 0 days. The minimum interval between doses is 4 weeks. Vaccine effectiveness against severe gastroenteritis caused by vaccine genotypes is reported as 98% (95% confidence interval 88% to 100%) for RV5, and 85 % (95% confidence interval 72% to 92%) for RV1. Children with severe combined immunodeficiency and history of intussusception cannot receive this vaccine. Further studies are needed to evaluate the safety and effectiveness of rotavirus vaccines among transplant recipients; however, to date, there are no known vaccine-derived rotavirus infections in either SOT or HSCT recipients. However, family and household member of these patients can and should be vaccinated. Highly immunocompromised individuals should avoid handling diapers of infants for 4 weeks after rotavirus vaccination.
Diagnosis
Rotavirus can be detected in stool samples using enzyme immunoassay, latex agglutination, and nucleic acid testing, such as PCR. Enzyme immunoassays are widely used and have high sensitivity and specificity. PCR-based tests are rapid, specific, and highly sensitive and can detect viral shedding for a longer period than enzyme immunoassay. Immunocompromised children with unexplained diarrhea should be tested for viral agents of gastroenteritis including rotavirus. Multiplex PCR assays may be preferred for their increased sensitivity and ability to detect multiple viral agents.
Treatment
Similar to other causes of viral gastroenteritis, supportive measures remain the primary treatment for rotavirus infection. Currently there are no FDA-approved antiviral agents for the treatment of rotavirus infections. Enteral administration of immunoglobulin has been used. Nitozoxanide has some in vitro activity for rotavirus. The clinical efficacy of both therapies remains unclear and data are limited. In a retrospective study of 41 episodes of rotavirus infection in pediatric HSCT recipients, the median duration of clinical symptoms after initiation of nitazoxanide was shorter (11 days [range 2 to 85 days]) compared with those who received enterally administered immunoglobulins (23 days [range 10 to 107 days]) or a combination of both treatments (26 days [range 6 to 90 days]), but was similar to those who received no treatment ( P = .1). No adverse effects of either treatment were documented. In a single-center study including four pediatric HSCT patients with confirmed rotavirus infections, median time from initiation of enteral immunoglobulin to symptom resolution was 3 days, and stool frequency and consistency were improved compared with historical controls. Additional studies are necessary before any of these can be considered for routine use.
Infection prevention and anticipatory guidance
Contact precautions are indicated for children with rotavirus during the duration of diarrhea. Soap, water, and bleach solutions can be used to prevent transmission through environmental surfaces.
Astrovirus
Astrovirus infections are among the most common causes of gastroenteritis in children. Astroviruses are small, non-enveloped, positive-sense single-stranded RNA viruses in the family Astroviridae . Astroviruses have a distinctive surface star-like appearance under electron microscope. The family is divided into two genera; the genus Mamastrovirus infects mammals and the genus Avastrovirus infects poultry. There are at least eight distinct serotypes of human astroviruses, with serotype 1 viruses detected most commonly.
Epidemiology and risk factors
Human astrovirus infections affect predominantly children, particularly those younger than 2 years. Elderly and immunocompromised hosts are also at risk for astrovirus infection. Astrovirus is transmitted predominantly through the fecal-oral route, although ingestion of contaminated food and water and contamination of surfaces may play a role. Fresh produce washed with contaminated water has been implicated in outbreaks. Most of the published outbreaks have occurred in closed populations of younger children and elderly, such as in child care centers or hospitals. Globally, human astroviruses are estimated to cause 2 to 9% of cases of acute, nonbacterial diarrhea requiring hospitalization in children. Mendez-Toss and colleagues reported that 4.6% of stool samples collected from Mexican children younger than 5 years with diarrhea had positive findings for human astroviruses. Astrovirus circulates year-round but the incidence peaks in cold weather periods in temperate regions. Viral shedding lasts a median of 5 days after onset of symptoms; however, it can be prolonged to several weeks in healthy children and persistent shedding may occur in immunocompromised patients. ,
Clinical manifestations
Human astrovirus infections often cause self-limiting diarrhea in immunocompetent individuals, but they can also disseminate to organs beyond intestines and cause severe infections in immunocompromised patients. The mean incubation period is 3 to 4 days. In immunocompetent children, astrovirus infections usually present with a mild, watery diarrhea that lasts 2 to 3 days, associated with vomiting, anorexia, abdominal pain, and sometimes fever. Vomiting and diarrhea are usually milder in astrovirus infection than rotavirus infection. Asymptomatic infections are common. In a recent study from Mexico, 2.6% of stool samples collected from children younger than 5 years without diarrhea were positive for human astrovirus using an enzyme-linked immunosorbent assay. Severe disseminated lethal infection and prolonged viral shedding have been described in immunocompromised patients. , Wunderli and colleagues described three pediatric recipients of HSCT with disseminated astrovirus infection with meningoencephalitis and chronic diarrhea; one infant died. Astrovirus RNA was detected in the respiratory tract, blood, bone marrow, skin, and brain. Astrovirus RNA has been detected in premature infants with necrotizing enterocolitis, but the causal association is uncertain.
Disease prophylaxis and prevention
No vaccine for astroviruses currently exists and, to our knowledge, none is under investigation.
Diagnosis
It is not possible to distinguish gastroenteritis caused by astrovirus infection from cases caused by other viral pathogens based on clinical presentation. Enzyme immunoassays have been used in epidemiologic studies to detect human astroviruses in stool specimens, and although they cannot identify the serotypes, they are helpful for rapid detection. Several FDA-cleared multiplex nucleic acid–based assays can detect astrovirus and have good sensitivity.
Treatment
Supportive treatments, including maintaining adequate hydration, nutrition, and electrolyte balance, are the mainstays of therapy. Currently there are no specific antiviral medications with FDA-approved labeling for the treatment of astrovirus and few data on potential strategies.
Infection prevention and anticipatory guidance
General measures for control of diarrheal infections, such as training care providers on general sanitation measures, hand hygiene practices, keeping food preparation areas separate from child care activities, and maintaining cleanliness of environmental surfaces using soap, water, and bleach solutions, can be used to prevent transmission. Symptomatic patients should have contact and standard precautions. Sick health care workers should be excluded from work until they are asymptomatic.
Sapovirus
Sapoviruses cause acute gastroenteritis in both humans and animals. Sapovirus is in the family Caliciviridae but is a separate genus from norovirus. Sapoviruses are genetically and antigenically diverse. They are small (30 to 38 nm), non-enveloped, single-stranded RNA virus. Five genogroups exist, and nine additional genogroups have been proposed. To date, only four genogroups (GI, GII, GIV, and GV) have been found to cause disease in humans.
Epidemiology and risk factors
The initial human outbreak of sapovirus was described at an orphanage in Sapporo, Japan, in 1977. Commercially available testing has only recently been available. Sapoviruses, like noroviruses and other human caliciviruses, are transmitted via fecal-oral route. Sapoviruses have been isolated from animals and contaminated water and food, including shellfish; foodborne and environmental transmission has been reported. Sapoviruses infect all age groups worldwide. Most cases are probably sporadic, but outbreaks have been reported associated with child care centers, hospitals, schools, and other group care settings.
Globally, sapoviruses cause a substantial proportion of pediatric diarrhea. A recent reanalysis of a multisite, international study (MAL-ED [Malnutrition and Enteric Disease Study]) using molecular diagnostic methods found that sapoviruses had the second and third greatest attributable incidence for diarrhea in children 12 to 24 months and younger than 12 months, respectively. In recent studies in the United States, sapovirus was isolated from the stools of approximately 5% to 10% of children with gastroenteritis , and 3% to 6 % of healthy, asymptomatic children. , Although sapovirus disease occurs year-round, the highest prevalence of sapovirus has been found in cold months, although studies have shown differences in seasonality.
Sapovirus also causes problematic infections in immunocompromised patients, although data are limited. A study among pediatric oncology patients with diarrhea isolated sapoviruses in 5% of the study population. Another study of immunocompromised patients found sapoviruses in 2% of symptomatic patients, although this study included adults and a large percentage of primary immunodeficiency patients.
Clinical manifestations
The clinical symptoms of acute gastroenteritis caused by sapoviruses are similar to those of other viral causes of gastroenteritis and include low-grade fevers, vomiting, nonbloody diarrhea, abdominal pain, and cramping. Sapovirus gastroenteritis may cause milder symptoms compared with norovirus and may cause more vomiting than diarrhea. Among immunocompromised patients, disease may be more severe. One study of immunocompromised patients with gastroenteritis cited a similar percentage requiring hospitalization (56%) compared with other viral causes, although numbers were small. Prolonged fecal excretion and diarrhea have been described in a renal transplant patient with sapovirus. A larger, more recent study of sapovirus infection in immunocompromised patients did not find any with chronic excretion.
Disease prophylaxis and prevention
No vaccine for sapoviruses currently exists and none is known to be under investigation.
Diagnosis
Antigen detection methods exist, but as for norovirus, these tests generally have low sensitivity owing to antigenic diversity. Reverse-transcription PCR is the diagnostic method of choice because of its excellent sensitivity and specificity and is available in both single-pathogen and one multipathogen testing platform. Of the three commercially available multipathogen testing platforms, only one includes sapoviruses. Therefore published data regarding sapovirus are somewhat limited compared with other viral causes of gastroenteritis. Because sapovirus is found in the stools of asymptomatic individuals and the PCR-based diagnostic testing has high sensitivity, a positive test result must be interpreted in the context of the clinical scenario.
Treatment
Supportive treatments, including maintaining adequate hydration, nutrition, and electrolyte balance, are the mainstays of therapy. No approved medications for sapovirus currently exist, and no published data exist regarding potential treatment in immunocompromised children. Nitazoxanide might have theoretical activity as for norovirus, but no data exist.
Infection prevention and anticipatory guidance
Prevention consists of general sanitation measures, including handwashing with soap and water and use of bleach-based disinfectants. Because of the similarity of sapoviruses and noroviruses, approaches to prevention and infection control are based on data for norovirus. Guidelines recommend standard precautions for all viral gastroenteritis with the use of contact precautions for diapered or incontinent patients for the duration of illness. Owing to the description of nosocomial outbreaks and the potential for serious consequences among transplant patients, we recommend contact precautions for all transplant patients with viral gastroenteritis and droplet precautions if vomiting. Sick health care workers should be excluded from work until they are asymptomatic.
Adenovirus
Human adenoviruses are a large group of double-stranded DNA viruses, which cause a broad spectrum of clinical disease in both healthy and immunocompromised individuals. In one study of children younger than 5 years in the United States with viral gastroenteritis, adenovirus was the third most common cause. Similarly, it is an important cause of gastrointestinal illness in immunocompromised children as well, isolated from 15% of pediatric oncology patients with diarrhea. Although adenovirus gastroenteritis is generally a self-limited disease in healthy children, it can be associated with more prolonged illness and disseminated disease in children who have undergone HSCT or SOT. For a complete discussion of adenovirus disease in immunocompromised children, please see Chapter 22 .
Conclusion
Diarrhea is a frequent and potentially debilitating condition in immunocompromised pediatric patients. The pathogens causing infectious diarrhea in immunocompromised children are similar to those causing disease in healthy children; however, symptoms can be prolonged and systemic and/or disseminated disease may occur. With increasing availability and use of molecular diagnostic testing, especially multiplex PCR-based tests, diagnosis of specific pathogens in viral gastroenteritis is increasing, leading to improved epidemiologic data on the viral causes of gastrointestinal illness in immunocompromised children. Because of the severity, multiplex PCR-based tests may be an important tool for immunocompromised children with diarrhea. Although the mainstay of therapy is supportive care and immunosuppression reduction when feasible, antiviral medications are being studied in this population, but more data are needed. Vaccine has been successful for prevention of rotavirus, and vaccines targeted against norovirus are under investigation, but additional vaccines for other viral causes of gastroenteritis are needed.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree