Disease
Affected population
Clinical manifestations
Laboratory diagnosis
Treatment
Chromoblastomycosis
(also called chromomycosis, verrucous dermatitis, figueiraa)
Usually in men aged 30–50 who work as farm laborers, lumberjacks, or sellers of farm products; affected persons usually poor, without adequate protective footwear and clothing
Slowly progressive disorder usually limited to the skin and subcutaneous tissue in which initial erythematous papular lesions may gradually evolve to varying morphologies, such as nodular, tumoral (cauliflower-like), plaque, verrucous, and cicatricial lesions; affects feet and legs most frequently; may transform into squamous cell carcinoma
Microscopic finding of muriform cells (sclerotic bodies) is the hallmark of this disease. Examination of scrapings or vinyl adhesive tape preparations, wet mount, histology, culture
Surgery effective in early stages; itraconazole (200–400 mg/day), terbinafine (250–500 mg/day), terbinafine (500 mg/day) plus itraconazole (200 mg/day); combination therapy (itraconazole with terbinafine or 5-flucytosine) for severe cases, posaconazole (400 mg bid) in patients with disease refractory to itraconazole or who are intolerant of itraconazole; cryotherapy
Eumycetoma
(also called mycetoma, maduromycosis, Madura foot)
Men aged 20–40 who work as herders, farmers, or other field laborers; increasingly in travelers to tropical endemic areas
Local chronic, progressive, multifistulous, suppurative, tumoral lesions discharging grains. Infection involves cutaneous and subcutaneous tissues, fascia, and eventually muscle and bone
Observation of grain color and texture; deep surgical biopsies containing grains that can be cultured or fixed for histopathology; immunodiffusion, ELISA, PCR with DNA sequencing; MRI or CT to determine bone involvement
Surgery and antifungal therapy with itraconazole (400 mg/day); posaconazole (400 mg bid) in patients with disease refractory to itraconazole or who are intolerant of itraconazole
Entomophthoramycosis
(also called subcutaneous zygomicosis, subcutaneous phycomycosis, basidiobolomycosis, conidiobolomycosis)
Infections usually caused by Entomophthorales, Basidiobolus ranarum, and Conidiobolus coronatus; usually in immunocompetent persons; basidiobolomycosis usually in children, conidiobolomycosis usually in adults
Basidiobolomycosis : usually has chronic progressive clinical course; hard nodules that spread, often over thighs and buttocks, eventually ulcerating overlying skin
Conidiobolomycosis : begins with swelling of inferior nasal turbinates and extends to facial and subcutaneous tissues and paranasal sinuses; eventually, subcutaneous nodules attach to underlying tissues, causing facial disfigurement
Histology—wide, sparsely septated, thin-walled hyphae with right-angle branching. Splendore-Hoeppli phenomenon present with basidiobolomycosis; culture
No standard treatment defined for entomophthoramycosis (basidiobolomycosis or conidiobolomycosis).
Antifungals used to treat entomophthoramycosis include potassium iodide, miconazole, ketoconazole, itraconazole, fluconazole, terbinafine, and amphotericin B
Lacaziosis
(also called lobomycosis, Lobo’s disease, Paracoccidioidomycosis loboi)
Men aged 21–40 who live in tropical rain forests and work as farmers, miners, hunters, and rubber workers
Very slow evolution over many years; small papules or pustules that evolve into keloid-like lesions, which gradually increase in size; pinna of the ear most commonly affected; original lesion followed by involvement of other areas by subsequent abrasion/autoinoculation; nodule distribution follows lymphatic system
Direct microscopy of tissue smear from lesion, examination of vinyl adhesive tape preparation; cannot be cultured
Serologic tests : have high sensitivity but lack specificity because of antigenic cross-reactivity with Paracoccidioides
Wide surgical excision, electrodessication in early stage of disease, cryosurgery; clofazimine (300 mg/day until clinical improvement, then 100 mg/day for ≥ 2 years)
Amphotericin B, 5-fluorocytosine, and azoles usually ineffective, but patient with disseminated disease undergoing treatment with itraconazole (200 mg/day)
Typically, these infections are characterized by initial lesions starting at the site of fungal implantation and with time, they be evolve according to the etiologic agent and the host immune defenses. Although the term “subcutaneous mycoses” have been used for decades, it is not strictly correct because in some of these infections the lymphatic vessels, fascia, muscles, cartilage, and bones may be affected beyond the cutaneous and subcutaneous tissues [1, 2].
Chromoblastomycosis
Chromoblastomycosis or chromomycosis is one of the most prevalent implantation mycosis in tropical and subtropical zones as well as the most frequent human mycoses caused by melanized (dark pigmented) fungi [7, 9–13]. This disease was described by Max Rudolph, a German Doctor working in Brazil, in 1914 [14, 15]. Chromoblastomycosis lesions are clinically polymorphic and if not recognized at earlier stages, they may become recalcitrant to therapy and extremely difficult to eradicate [8]. Except for the initial lesions, which should be surgically removed, moderate-to-severe clinical forms constitute a true therapeutic challenge for patients and clinicians. This disease presents with the following characteristics: primary lesion beginning at the site of implantation of the etiologic agent, chronic involvement of cutaneous and/or subcutaneous tissues, associated with a granulomatous, purulent, and fibrotic tissular reaction, and a non-protective humoral and cellular immune responses [7, 16].
Etiology
Melanized, dematiaceous, pheoid, or simply “black fungi” are ubiquitous saprobes inhabiting soil, plant fragments, and water. More than 150 species from 70 genera have been implicated in human and animal disease [17, 18]. These agents contain melanin in their cell walls, which serves a key role in virulence in black fungi as a group. The list of human infections related to the melanized fungi includes PHM, allergic and invasive sinusitis, fungemia, mycetoma, and CBM. The exact number of melanized (dematiaceous) fungi which may cause CBM is uncertain, especially as molecular tools continue to add to fungal taxonomy [13, 18–22]. It is believed that several species of the Herpotrichiellaceae family cause the disease. The main species, according to the taxonomic proposal, can be grouped into five genera: Fonsecaea, Cladophialophora, Phialophora, Rhinocladiella, and Exophiala. The majority of infections are caused by species of Fonsecaea and Cladophialophora [18–22].
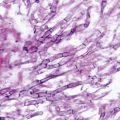
Similar to the endemic fungi pathogens, the agents of CBM are dimorphic and present their mycelial form in natural habitats or in vitro culture media, but in tissues present as muriform cells (the hallmark of this disease). Muriform cells, also known as “sclerotic or fumagoid cells,” “Medlar bodies,” or “copper pennies,” are a biological adaptation leading the agents to survive in the hostility of the host tissue environment [23, 24]. Muriform cells are characterized by thick melanized cell walls, isodiametric expansion, and unordered septum formation. They are polyhedric in shape, measuring 5–12 µm in diameter and divide by binary fission, forming two distinct planes of septation (Fig. 22.1). These are considered to be protective structures which also enhance resistance to antifungal drugs. The presence of muriform cells differentiated CBM from PHM [7, 9, 24].
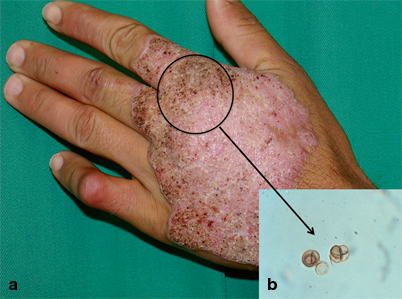

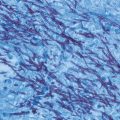
Fig. 22.1
A nodular plaque lesion of chromoblastomycosis containing several “black dots” (circle) was scraped (a). Muriform cells are easily observed in KOH wet mount (b). (*From Queiroz-Telles F, et al. Subcutaneous mycoses. Infect Dis Clin N Am 2003;17(1):59–85. Reprinted with permission from Elsevier Limited). KOH potassium hydroxide
In culture media, the agents of CBM grow as dark pigmented, velvety filamentous moulds. Presumed species identification can be made by conventional mycological methods, like macro- and micromorphology and physiology characteristics, but nowadays, the definitive agent identification should be based on the molecular sequence of specific genes. Although the fungal species causing CBM do not differ in clinical aspects, they may present differences in their in vitro susceptibility to antifungal drugs. Cladophialophora carrionii and Phialophora verrucosa are considered to be more susceptible to antifungals than Fonsecaea pedrosoi. Even differences in susceptibility have been reported between the species of Fonsecaea genus, viz. F. pedrosoi, F. monophora, and F. nubica [21–25].
Ecology and Epidemiology
Melanized fungi thrive in tropical and subtropical environments worldwide. It is believed that during their saprobic cycle, fungal agents of CBM live in soil and in different parts of plants such as thorns, leaves, spiny seeds, wood cortex, etc. [18, 26, 27]. Consequently, CBM lesions afflict mainly adult males involved in rural labor who do not have appropriate footwear or clothing and who are more likely to be infected by trauma. In 54–85 % of cases, CBM involves the lower extremities [13, 26]. It is considered as an occupational disease in the endemic areas of the world, but scattered cases are also reported in temperate-to-cold regions. Although CBM has no compulsory notification, gathered data from published case reports and surveys show that incidence rates may range from 1:6800 (Madagascar) to 1:8,625,000 (USA) [10, 28]. Most of the reported cases occur in Latin America, the Caribbean, Asia, Africa, and Australia. Madagascar, Brazil, Mexico, Dominican Republic, Venezuela, India, and Southern China contribute with the majority of cases. The disease is less frequent in the Northern Hemisphere, including European countries and in the USA, where it has been reported in Louisiana and Texas [2, 4, 5, 7].
Although children may present with the disease, the majority of patients are adult males. The main risk factors associated with CBM infection are adult age, male sex, rural work or outdoor activities, the lack of protective shoes, gloves or garments, and poor nutrition and hygienic habits [2, 26]. In a series of 100 patients reported from the Brazilian South Region, the majority of patients were male (4:1) of 50–59 years of age [29]. In another study of 325 cases in the Brazilian Amazon region, the main age group affected by the diseases ranged from 41 to 70 years old, 86.1 % of the patients were agricultural workers, and 93.2 % of were male [11]. In both reports, CBM lesions predominated on the lower limbs (feet and legs) and F. pedrosoi was the main etiologic agent [11, 29]. On the other hand, a series of cases in children and adolescents patients ranging from 2 to 19 years old have been reported in an endemic Venezuelan semiarid zone. Infection was related to C. carrionii, probably transmitted by cactaceae thorn implantation [30].
Immunopathogenesis
Human infection by CBM agents starts when fungal elements are transferred from their environmental saprobe life across the cutaneous barrier through transcutaneous trauma. If the parasite survives and adapts to the new hostile environment represented by the host’s cutaneous/subcutaneous tissues, the infection may progress to disease [2, 16]. Because labor-related trauma is very frequent among rural workers living in tropical regions, unsuccessful infections may be the rule since melanized fungi are ubiquitous in nature, but CBM is not an everyday disease (even in the endemic areas). So other factors must play a hole in the pathogenesis of CBM, including fungus virulence, host genetic susceptibility, and continuing exposure to the agents during life. It has not been proved if hormonal factors are able to protect females like in paracoccidioidomycosis [16, 27, 31 – 33].
The knowledge of immunology of CBM is poorly understood because, to date, there are no reproducible animal models of this disease. Mechanisms of immunity may include humoral and cell-mediated responses. Like other chronic fungal infections, CBM patients produce specific antibodies, but not protective antibodies against the progression of the disease. It has been demonstrated that the cell-mediated immunity plays a key role in the clinical presentations of this disease. The mixed tissue response in CBM shows a granulomatous reaction associated with microabscesses, suggesting ineffective phagocytosis of muriform cells and chronic infection [34, 35].
Clinical Manifestations
Following implantation, and after an uncertain period of time, the initial lesion may be produced at the site of infection. It may start as a solitary macular lesion and latter, it may progress to a papular shape lesion with a pink smooth surface that gradually increases over a few weeks and then develop a scaly surface. The initial skin lesion may progress and evolve with diverse clinical polymorphism, eliciting differential diagnoses including many infectious and noninfectious diseases (Table 22.2). According to the modified Carrion’s classification, the CBM lesions are characterized as nodular, tumoral (cauliflower-like), verrucous, cicatricial, or plaque (Fig. 22.2) [7, 13, 24]. In advanced and more severe cases, more than one type of lesion can be observed in the same patient. The lesions may also be graded according to severity, which may help clinicians plan a patient’s therapy (Table 22.2) [7, 13, 28]. Initially, the mild form lesions are asymptomatic, but with time itching becomes the predominant symptom of the disease, which in the moderate forms is intense and may be accompanied by local pain. As severity increases, edema and bacterial secondary infections may lead to limitation or incapacity to continue working. Because the CBM lesions are very pruritic, it is believed that disease dissemination usually occurs by autoinoculation and contiguous lymphatic spread [9, 13]. However, lymphatic dissemination has been reported in a few cases. In very advanced cases, chronic lymphedema, ankylosis, and malignant transformation may occur [7, 37, 38].
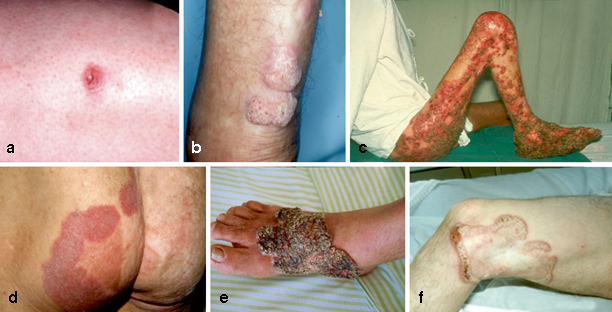

Fig. 22.2
Clinical classification of types of chromoblastomycosis lesions. Initial lesion (a), nodular (b), tumoral (c), plaque (d), verrucous (e) and cicatricial (f). (*From Queiroz-Telles F, et al. Subcutaneous mycoses. Infect Dis Clin N Am 2003;17(1):59–85. Reprinted with permission from Elsevier Limited)
Table 22.2
Lesion types, severity rating, and differential diagnosis of chromoblastomycosis
Type of lesions | Severity of disease | Differential diagnosis |
|---|---|---|
Nodular type Moderately elevated, fairly soft, dull-to-pink violaceous growth. Surface smooth, verrucous or scaly. With time lesions may gradually become tumorous | Mild form A solitary plaque or nodule measuring less than 5 cm in diameter | Infectious diseases Fungi Paracoccidioidomycosis Blastomycosis Fixed sporotrichosis Coccidioidomycosis Phaeohyphomycosis Lacaziosis Eumycetoma Granulomatous candidiasis Granulomatous trichophytosis Bacteria Cutaneous verrucous tuberculosis Leprosy Actinomycetoma, nocardiosis Botryomycosis Tertiary syphilis, yaws Ecthyma Mycobacteriosis (M. marinum, M. fortuitum) Protozoa Cutaneous leishmaniasis Rhinosporidiosis Viral Verruca, papilloma |
Tumorous type Tumor-like masses, prominent, papillomatous, sometimes lobulated; “cauliflower like.” Surface partly or entirely covered with epidermal debris and crusts. More exuberant on lower extremities. | ||
Verrucous type Hyperkeratosis is the outstanding feature Warty dry lesions. Frequently encountered along the border of the foot | Moderate form Solitary or multiple lesions: nodular, verrucous or plaque types, existing alone or in combination, covering one or two adjacent cutaneous regions, measuring less than 15 cm in diameter | |
Cicatricial type Non-elevated lesions that enlarge by peripheral extension with atrophic scarring, while healing takes place at the centre. Usually with annular, arciform or serpiginous outline. Tends to cover extensive areas of the body | ||
Plaque type Slightly elevated, with variously sized and shaped areas of infiltration. Reddish to violaceous in color presenting a scaly surface, sometimes shows marked lines of cleavage. Generally found on the higher portions of the limbs | Severe form Any type of lesion alone or in combination, covering extensive cutaneous regions whether adjacent or nonadjacent | Helmintic Filariosis Noninfectious diseases Escamous carcinoma Bowen disease Psoriasis Sarcoidosis Lupus erythematosus Mossy foot |
Diagnosis
As CBM lesions have a diverse morphology leading to an equally diverse differential diagnosis, this disease must always be confirmed by mycological exams (direct examination and culture) and/or histopathology [7].
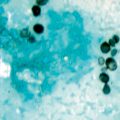
Muriform cells may easily be observed on direct exams of crusts, skin scrapings, aspirates, debris, or tissue fragments taken from the lesions after potassium hydroxide (KOH) (10–40 %) digestion. The sensitivity of the direct examination ranges from 90 to 100 %. Fungal elements are easily found at the lesional surface, resembling “black dots” (which are small hematic crusts with cellular debris and fungal structures), resulting from their transepithelial elimination (Fig. 22.1). Occasionally, near the cutaneous surface, these muriform structures may germinate and undergo dimorphic transformation into filamentous fungal forms [39, 40].
In histologic sections, CBM lesions are characterized by pseudoepitheliomatous epidermal hyperplasia, hyperkeratosis, irregular acanthosis, alternating with areas of atrophy and collections of inflammatory cells forming epidermic abscesses. Granulomatous reaction with different grades of fibrosis can be found at the dermal level. Muriform cells may be observed among these structures or inside Langerhans giant cells (Fig. 22.3) [41].
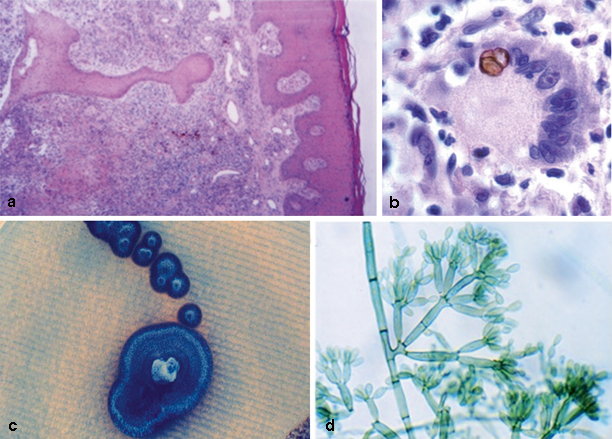

Fig. 22.3
Skin biopsy of patient with chromoblastomycosis with hyperkeratosis, acanthosis and pseudoepitheliomatous epidermal hyperplasia. The derma depicts a mixed inflammatory reaction containing granulomata and abscesses. HE × 100 (a). Granuloma detail showing a Langhans giant cell with a muriform cell. PAS × 1000 (b). Melanized colony on Sabouraud dextrose agar (c) and microscopic morphology of Fonsecaea pedrosoi conidiogenesis. Slide culture × 600 (d). *Courtesy of Vania Aparecida Vicente PhD, Curitiba, Brazil. HE Histology, PAS periodic acid–Schiff
In culture, all the CBM agents are slow-growing dark fungi. Initially, the colonies are deep green, developing a velvety dark aspect on their surface with time. Traditionally, their correct identification has been made through microscopic examination of the asexual reproductive structures like conidiophores, conidiogenous cells, and conidia (Fig. 22.3) [7].
Therapy
Over the century that has passed since Max Rudolph first reported the disease, several therapeutic regimens have been proposed, including physical therapeutic methods and chemotherapy with antifungals (Table 22.3). As comparative trials on this disease are lacking, evidence that helps to select optimal therapy is based on a few open clinical studies and many expert opinions. Though no “gold standard” therapy for CBM has been identified, several treatment options are available. Except for its initial localized lesions, which may be treated with surgical excision, most of the CBM clinical forms require long-term continuous systemic antifungal therapy. The duration of therapy may range from months to years and even more than one decade.
Table 22.3
Treatment options for chromoblastomycosis
Physical methods | Chemotherapy | Combination therapy |
|---|---|---|
Standard surgerya Photodynamic therapyc Cryotherapyc Iontophoresise Moh’s surgerye CO2 lasere Local heat (dry)e | Itraconazoleb Terbinafineb Posaconazoled Amphotericin Be 5-fluorocytosinee 5-fluorouracile Thiabendazolee Calciferol (Vit D3)e Ketoconazolee Fluconazolee | Itraconazole + terbinafinb Itraconazole + 5-fluorocytosined Itraconazole + cryotherapyd Terbinafin + cryotherapyd Itraconazole + photodynamic therapyd Itraconazole + imiquimodd |
Based on noncomparative trials, patients are mostly treated with itraconazole 200–400 mg per day, terbinafine 500 mg per day, or with a combination of both drugs in refractory patients. The combination of itraconazole (200–400 mg daily) with 150–200 mg/kg per day of 5-flucytosine may also be successful in recalcitrant cases [42–49]. There are several reports indicating that physical methods, including photodynamic or thermo therapy (topical cold or heat), may shorten the needed duration of systemic antifungal treatment [50–55]. It is important to emphasize that physical methods must be used in combination to antifungal drugs. Among the new triazolic drug, posaconazole has achieved favorable results in severe refractory cases of CBM, while voriconazole and isavuconazole have not been effectively tested [56, 57].
Mycetoma
The term mycetoma derives from the Greek terms mykes (fungus) and oma (tumor). The disease was first mentioned in Atharva Veda, an ancient Indian religious book, as pada valmikan, which means foot anthill. The oldest known case of mycetoma most likely dates from the Byzantine period, as evidence was found in a human skeleton based on the bone morphological characteristics [58]. However, the disease was first described by John Gill in a dispensary report of the Madras Medical Service of the British Army in India in 1842, where it was named “Madura foot,” as many cases originated from the city of Madurai [59]. The first description of mycetoma in the medical literature was given by Godfrey in 1846, who called it morbus tuberculosis pedis [60]. The fungal nature of the disease was established by Vandyke Carter, who also established several features, including its natural history, the body parts that are most commonly affected, the grain colors, the presence of bone destruction, and the higher incidence among males [61]. It was also named mycetoma in 1860, which is the term by which it is currently known. In 1913, Pinoy subdivided the disease as a function of its bacterial or fungal etiology into two categories, actinomycetoma and eumycetoma, respectively [62].
Mycetoma is known under various names, such as fungus disease of India, Godfrey and Eyre’s disease, endemic degeneration of the bones of the feet, morbus pedis entophyticus-affection singulière, and perforating ulcer of the foot.
Mycetoma has long been a neglected infection because of its rarity and geographical isolation in poor communities. In 2013, the World Health Organization (WHO) accepted the disease as the first fungal neglected disease, and this will promote substantial improvements in care for these stigmatized patients [63–65].
The true incidence of mycetoma is unknown due to its slow and chronic natural history and late presentation. Nevertheless, together with sporotrichosis, eumycetoma is the subcutaneous mycosis most frequently described. It exhibits global distribution, being most common in tropical and subtropical countries between the Tropic of Cancer and the Tropic of Capricorn. While actinomycetoma occurs more frequently in Central and South America, eumycetoma is most commonly found in Africa and in India [66, 67]. Approximately, 60 % of the cases of mycetoma worldwide are caused by filamentous bacteria, while the remainder of the cases are caused by fungi. However, there are regional variations, and the rate of eumycetoma in Yemen is 73 %. In fact, the prevalence of eumycetoma is high in the so-called mycetoma belt, which extends between latitudes 15° South and 30° North and includes Sudan, Mauritania, Somalia, Senegal, Egypt, Nigeria, Niger, Kenya, Ethiopia, Chad, Cameroon, Djibouti, India, and Yemen. In addition, the disease is endemic in Mexico, Venezuela, Colombia, and Argentina, and cases may also be found in temperate countries, such as Mediterranean countries, including North Africa, Greece, and Italy [2, 4, 5, 68]. Cases affecting travelers to endemic areas are frequently reported. The areas with high prevalence of mycetoma are fairly arid and have a short rainy season, lasting 4–6 months, annual precipitation of 50–1000 mm, relative humidity of 60–80 %, and a constant temperature of 30–37 °C during the night and day. The rainy season is followed by a dry one that lasts 6–8 months, with 12–18 % relative humidity, a daytime temperature of 45–60 °C and a nighttime temperature of 15–18 °C. Such extreme temperature variation might be a requisite for the survival of the etiologic organisms in their natural niches. Within the mycetoma belt, the largest number of cases occurs in Sudan, where the disease is particularly endemic and highly disabling. According to some studies, 300–400 new cases occur in Sudan every year [65, 69, 70].
Mycetoma is also highly endemic in Mexico, with an average of 70 new cases per year, most of them caused by bacteria. The ecology of the various etiological agents of mycetoma might be currently changing in the Americas, as in Brazil the ratio of actinomycetoma to eumycetoma is 1:1. In Argentina, most cases are classified as eumycetoma. Ecological factors clearly determine the geographical distributions of the etiological agents. However, the considerable displacement of large populations and the ease with which people currently move from one place to another also contribute to the changes in the pattern of disease [2, 4, 66]. A few cases of mycetoma have been reported in the USA [70].
Mycetoma more frequently affects individuals who encounter frequent and direct contact with the soil, especially those in rural areas, such as farmers and herdsmen. That fact notwithstanding, the disease is not exclusive to those individuals, as cases among urban workers, homemakers, travelers, members of humanitarian organizations, and archeologists have also been reported [63–66, 71].
The disease is known to mostly affect men, most likely as a function of their greater involvement in rural activities. Most affected women are also rural workers. These facts notwithstanding, a recent study suggested that the progesterone levels exhibited by women might inhibit the growth of some etiological agents of mycetoma, such as Madurella mycetomatis.
Mycetoma occurs more frequently among individuals aged 20–40 years, whereas in endemic areas, it might also affect children and older adults [2, 4, 63, 66].
Etiology
More than 30 different species of fungi have been associated with eumycetoma, some of which are able to cause a broad range of diseases, including PHM and CBM [2, 58, 66, 72].
The etiological agents of mycetoma depend on factors such as temperature, precipitation, type of soil, vegetation, and the demographic characteristics of the susceptible population. These agents are classified according to the color of the grains, which might be black, yellow, or white. The grains are also known as sclerotia and consist of aggregates of fungal hyphae embedded in a hard concrete-like matter [58, 71].
Table 22.4 summarizes the main etiological agents of eumycetoma. More than 90 % of cases of eumycetoma reported worldwide are caused by just four agents: Madurella mycetomatis, Madurella grisea, Leptosphaeria senegalensis, and Pseudallescheria boydii, the former three being melanized fungi and the latter a hyaline one [7, 58].
Table 22.4
Main features of eumycotic and actinomycotic grains
Eumycetomaa | Fresh examination | Histology (HE) |
|---|---|---|
Scedosporium apiospermum | < 2 mm, yellowish or white, soft, oval to lobed, “fig seed like” | Compact, no cement, interwoven hyaline hyphae < 5 µm and swollen cells < 20 µm, eosinophilic border |
Acremonium kiliense | < 1.5 mm, white, soft, irregular shape | Compact, no cement, hyaline hyphae < 4 µm, swollen cells < 12 µm |
Aspergillus nidulans Fusarium moniliforme | < 2 mm, white, soft, oval to lobed | Compact, no cement, interwoven hyaline hyphae < 5 µm, eosinophilic border |
Neotestudina rosati | White to brownish, soft, < 1 mm, fragmented angulated mass | Cement and swollen cells at periphery embedded in cement and some central vesicles |
Madurella mycetomatis | < 2 mm, black, firm to brittle (coal consistence), oval to lobed | Compact type, with brown-staining cement
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|