3.1 Introduction
All plant and animal cells are bounded by barriers that provide protection from the outside world and thus help to prevent infection. In the case of multi-cellular organisms there is a new problem: they can potentially be infected anywhere in the organism, so how can they ensure that the mechanisms needed for defence against infection are available wherever it occurs? One way is to have the defence mechanisms located throughout the entire body at all times, but this would be inherently inefficient. Another solution has evolved.
Most multicellular animals – certainly those that are more complex – possess mechanisms for moving cells and molecules around the body. Thus these can be used for bringing defence-related cells and molecules to the sites of infection and damage so that the infectious agents can be eliminated and tissues can be repaired. These recruited mechanisms are found in one form or another in all complex organisms. We know these as innate defences or innate immunity. However complex organisms first arose about half a billion years ago, during the Cambrian explosion and, starting with the earliest fish, it is possible to trace the evolution of specialized tissues and organs that reflect the emergence of an entirely new form of immunity. This is a adaptive immunity, which is mediated by lymphocytes.
In this chapter we examine the anatomical and physiological basis of innate and, adaptive immunity, and show how this relates functionally to defence against infection. We first consider the anatomical, physiological and chemical barriers that provide the first line of defence against infection, and which need to breached before infection can occur (Section 3.2). Infection can occur at any site in the body, and we next discuss the changes induced locally by infection that result in inflammation. Inflammation allows the cells and molecules of innate immunity to be targeted to infected sites, enabling the immediate and later recruitment of effector mechanisms that may help to eliminate the infectious agent and, afterwards, to heal the damage that has occurred. We show how this local response also drives the production of molecular messages that induce physiological responses in other tissues and organs at more distant sites (Section 3.3).
We then turn to the tissues and organs involved in adaptive immunity, the lymphoid tissues (Section 3.4). We describe how lymphocytes and other cells continually traffic though these organs, how lymphoid tissues become modified when infection occurs, and how lymphocytes respond in these sites to infectious agents. The lymphocytes then generate new effector mechanisms that can help to eliminate infection and later provide “memory”, a feature unique to adaptive immunity that provides increased protection if the same pathogen is encountered in the future.
We next turn to where these immune cells are produced, examining the specialized tissues and organs that contain the haematopoietic stem cells (HSCs) and later progenitors that differentiate into the cells of innate immunity and lymphocytes (Section 3.5). We discuss some of the factors that control the development of these tissues, and their pathological induction at other sites in disease. Finally, we touch on how bone marrow stem cells can be used to treat immunodeficiencies and how, potentially, they can be genetically modified to develop treatments for immunological and other defects (Section 3.6).
3.2 Natural Barriers
The first line of defence against infection consists of barriers, which may be structural, chemical or biological. Examples are keratin in the outermost layer of the skin, acid in the stomach and commensal bacteria in the intestine, respectively. These natural (as opposed to induced) defences are present in all normal individuals before any infection occurs and they normally need to be breached by any microbe that is going to cause disease. Their importance is shown where any of these barriers is defective, leading to a greatly increased risk of infection at these sites and beyond.
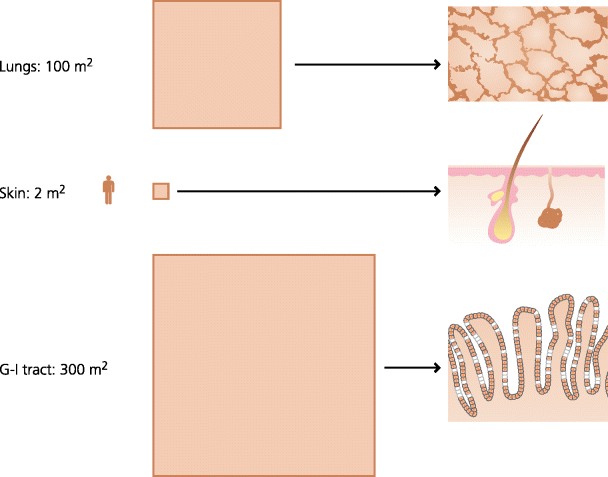
The skin is the most obvious barrier, but it is in fact the smallest of the surfaces in contact with the external environment, in contrast the surface areas of the lungs and the gastro-intestinal tract are respectively 50 and 150 times that of the skin. That these huge areas of tissues can be monitored effectively for infection is perhaps surprising and it explains why inflammation is needed to target defence mechanisms to specific areas when required (below). See Figure 3.1.
Fig. 3.1 Surface areas of skin and mucosal tissues. Mucosal tissues have many times the surface area of the skin. In adult humans the respiratory tract has a surface area of roughly the size of a tennis court and the gastro-intestinal (G-I) tract of a football pitch. These surfaces are also much thinner, often only one cell thick. It is not surprising that most infections start at mucosal surfaces.
3.2.1 Skin
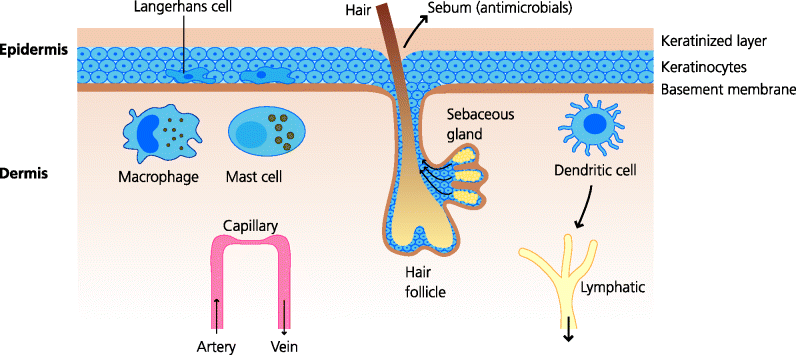
The skin has a major protective function, not only against infection but also against mechanical trauma. Thus, the outer layers are hard and difficult for microbes to penetrate because of the presence of much keratin, and this provides a barrier to infection. The importance of this is shown by diseases such as eczema where the skin is damaged: bacterial (e.g. staphylococcal) and fungal infections are common in the damaged areas. Similarly, where the skin is breached by a wound or a burn, bacteria can get into subcutaneous tissues which can be fertile grounds for them to thrive. Patients with serious burns are at severe risk of dying from massive infection, and also from the side effects of the inflammatory mediators that the immune system produces to try to counter the infection. Apart from this mechanical barrier function, skin secretions, such as sebum produced by the sebaceous glands, contain anti-microbial chemicals. The relative paucity of sebaceous glands in some skin areas, such as the feet, may relate to the increased incidence of fungal infections in these areas (e.g. athlete’s foot). See Figure 3.2.
Fig. 3.2 Structure of skin showing defence barriers. The epidermis varies in thickness in different parts of the body, but is always several cells thick and the outer layers are always keratinized, forming a mechanical barrier to infection. Sebaceous glands discharge their contents, including anti-microbial molecules, into the hair follicle and thence onto the surface of the skin. Both the epidermis and the dermis contain classical dendritic cells (DCs), Langerhans cells and dermal dendritic cells, respectively involved in the initiation of adaptive immunity. The dermis also contains macrophages and mast cells that can sense infectious agents that have breached the outermost layers.
3.2.2 Mucosal Surfaces
Mucosae are epithelial tissues that in general contain goblet cells, the source of mucus. The main mucosae are the gastro-intestinal tract, the respiratory tract and the urogenital tract, but the eye is also included. Given that the main functions of mucosae are transport (absorption and secretion) they could not function if they were keratinized. They are also often only one cell thick. Thus, pathogens have a much easier task if they are to penetrate mucosae. Mucosae do, however, have their own defence mechanisms. Some mechanisms are common to most mucosae. All mucosae need lubrication and in most cases this is mediated by mucus or, in the case of the eye, by tears. Mucus itself is thought to provide a mechanical barrier to infection and may carry anti-microbial molecules (e.g. defensins: see Chapter 4). However, children suffering from cystic fibrosis, where the mucus in the respiratory tract is abnormally thick, have an increased incidence of bacterial lung infections because microbes cannot be efficiently expelled from the lungs by coughing. Tears wash away pathogens but also contain lysozyme, a molecule capable of breaking down some bacterial cell walls.
3.2.2.1 Respiratory Tract
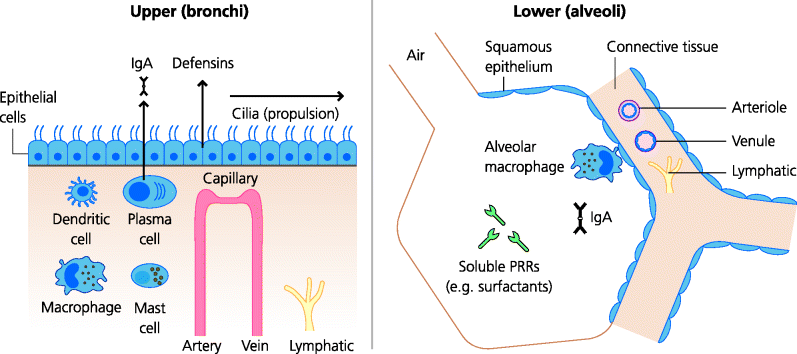
The epithelial cells of much of the respiratory tract are ciliated, and these cilia beat coherently to move secretions and particles towards the external world. In chronic cigarette smokers the cilia have disappeared and the epithelial cells are flattened (squamous metaplasia). Thus secretions containing bacteria pool in the lower parts of the tract and provide first-class growth environments for the bacteria. Lower down the respiratory tract, in the alveoli, are large numbers of macrophages, capable of phagocytosing and killing a range of microbes. Some of the lubricant surfactant proteins secreted into the lungs can also help to eliminate microbes. See Figure 3.3.
Fig. 3.3 Mucosal tissues I: respiratory tract. The upper respiratory tract (trachea and bronchi) is lined by ciliated epithelial cells – the cilia beat coherently to move secretions towards the mouth. The alveoli, where gas exchange occurs, are lined by flat, squamous epithelium. They contain alveolar macrophages that may have a defence function, but also clear inhaled particles. Other cells in the alveoli secrete molecules such as surfactants which are lubricants but may also have anti-microbial effects, acting as pattern-recognition receptors (PRRs), see Chapter 1. Some IgA may be produced as natural antibodies before adaptive immunity is triggered and may help to provide an extra layer of defence against infection of the epithelia.
3.2.2.2 Gastro-Intestinal Tract
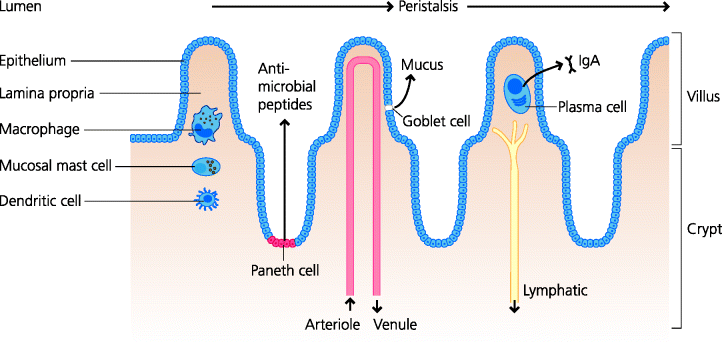
The gastro-intestinal tract possesses several kinds of barrier. Many microbes can be killed by acid. Hydrochloric acid is secreted into the stomach and its primary function is to aid digestion, but it can also kill many microbes. Hence, patients suffering from a deficit in stomach acid may be more susceptible to intestinal infections with bacteria such as Salmonella. Bile is secreted from the gall bladder into the upper small intestine. The main function of bile is also to aid digestion, but bile salts are also capable of killing many bacteria. Thus, culture media designed to isolate enteric bacteria from complex mixtures are formulated to contain bile salts because only the resistant intestinal bacteria can grow in these conditions. Bile salts can also destroy the envelopes of viruses, perhaps explaining why viruses that infect the intestine do not possess a lipid envelope. Peristalsis serves to drive out pathogens in the faeces (this may, however, be to the pathogen’s advantage in spreading infection) but many bacteria are able to bind to intestinal epithelial cells, preventing their expulsion. See Figure 3.4.
Fig. 3.4 Mucosal tissues II: intestine. The intestine is lined by a single layer of epithelial cells. In the small intestine villi and crypts are present. The large intestine has a smooth surface, but contains many deep tubular glands. Within the epithelium, goblet cells secrete mucus that is a lubricant, but also has mechanical barrier functions. In the crypts of the small intestine, specialized Paneth cells secrete a variety of anti-microbial peptides such as defensins. The lamina propria of the intestine contains macrophages and mucosal mast cells, classical DCs, usually eosinophils (not shown), and plasma cells that typically secrete IgA that is transported into the lumen. Peristalsis serves to propel the intestinal contents towards the anus.
3.2.2.3 Urogenital Tract
Bacterial cystitis, inflammation of the bladder, is very common in women, but very rare in men. Might this relate to the relative lengths of the ureters in the two genders – much longer in men – and to the anatomical juxtaposition of the urethra and anus in women? One survey of men and women travelling to work sampled their hands for faecal bacteria: more than 20% of those sampled carried faecal bacteria, and the incidence was higher in women than in men. Motile bacteria, such as Escherichia coli, also have much further to swim in men and tidal urine flow is more likely to wash them out. Not surprisingly, conditions leading to urinary obstruction often result in infections, illustrating the importance of this flushing effect of urine for basic defence.
3.2.2.4 Commensal Flora
Another important barrier to infection by pathogens is the commensal bacteria that coat many mucosal surfaces. Commensals are present in huge numbers in the large intestine. As we noted in the Introduction there may be about 10 times more bacteria in the human colon than all the cells that make up an average adult human body. Commensals also coat many other epithelial surfaces including the skin, the respiratory tract, and the urogenital tract of women. In most of us these bacteria do no harm and probably act to prevent pathogenic bacteria from causing disease: they form an effective barrier to colonization by pathogens. In some instances of large bowel surgery, patients used to be given large doses of oral antibiotics to get rid of most of the intestinal bacteria, so as to reduce the chance of bacteria infecting the peritoneal cavity. In such cases ingestion of the bacterium Staphylococcus aureus sometimes led to a serious condition known as enterocolitis in which parts of the wall of the large intestine were destroyed (necrosis), often leading to perforation and peritonitis that could be fatal. Commensals can also help to make the local environment more resistant to infection. For example, lactobacilli in the vagina produce acid that is thought to reduce the risk of bacterial or fungal infection. Indeed there is evidence to suggest that vaginal douching, by removing the lactobacilli, leads to a rise in pH, creating conditions in which other pathogens can thrive.
3.3 Functional Anatomy of Innate Immunity
The natural barriers, outlined above, are absolutely crucial as the first lines of host defence against infection. It is, however, almost a defining feature of a pathogen that it can avoid or evade these barriers. The next major line of defence is the innate immune system, and a phenomenon that is absolutely central for effective functioning of innate immunity (as well as adaptive immunity) is acute inflammation. Here, we will discuss the major principles of inflammation in relation to the structure and function of the tissues in which inflammation occurs. We also briefly introduce some of the cells and molecules that are involved, and which are discussed in more detail in Chapter 4.
3.3.1 Features of Inflammation
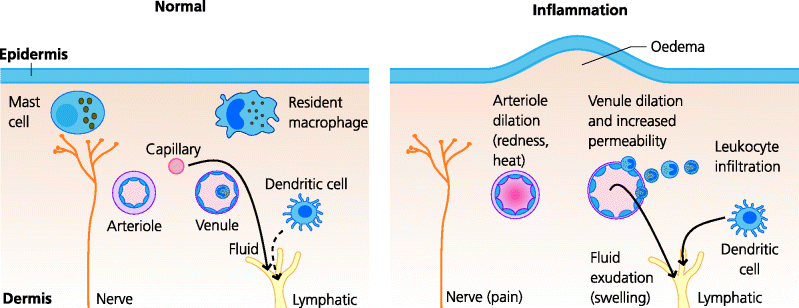
What is acute inflammation? Inflammation was defined by Menkin in the 1950s as “the complex vascular, lymphatic and local tissue reaction elicited in higher animals by the presence of micro-organisms or of non-viable irritants”. Inflammation is a process, not a state, and inflamed tissues are undergoing continual change. The cardinal features were recognized by the Greeks: redness and heat, reflecting dilatation of small blood vessels and increased blood flow; swelling, reflecting the increased accumulation of excess extravascular fluid, and which is called oedema; local pain, caused by increased tissue tension and the release of chemicals that stimulate pain nerve fibres; and, sometimes, loss of function. Acute inflammation is generally thought of as inflammation that occurs rapidly within minutes, hours or a few days of the initial stimulus and which subsequently resolves. In contrast, chronic inflammation has a time course of weeks, months or years. Chronic inflammation generally results from the persistence of the initiating inflammatory stimulus. It is important to realize that acute inflammation in a tissue may not be clinically apparent. In dealing with non-pathogenic microbes exactly the same defence mechanisms may operate that are used to deal with pathogens, but the local changes in infected tissues may be so minor as to be unapparent. These are called subclinical infections. See Figure 3.5.
Fig. 3.5 Acute inflammation in the skin. Acute inflammation induces vascular changes: dilatation of arterioles and venules and increased permeability of venules. This increases overall blood flow to the area, but also slows the flow in venules, increasing the probability of leukocytes attaching to the endothelium. Changes in the expression of venule adhesion molecules permit the recruitment of neutrophils and monocytes into the inflamed tissue. The increased permeability, due to gaps forming between endothelial cells, permits exudation of water and solutes including macromolecules such as antibodies and complement. The water causes swelling – oedema, one of the main features of acute inflammation; another is often pain.
3.3.2 Initiation of Local Inflammation
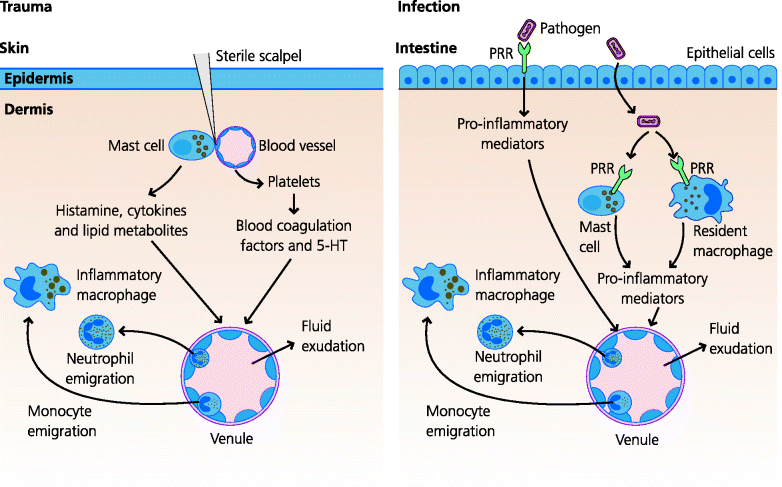
Inflammation has two distinct but overlapping and closely related functions in defence against infection. (i) By increasing the permeability of blood vessels, inflammation permits macromolecules involved in defence and healing to enter extravascular tissues. (ii) By increasing the adhesiveness of the blood vessel endothelium, it enables leukocytes to be recruited from the blood into the inflamed area. Thus, the requisite molecular and cellular effector mechanisms can be rapidly targeted to the site of infection. If, however, inflammation is to be initiated, the presence of tissue damage has to be recognized. Given the number of ways in which tissue damage can be caused, by trauma as well as infection, it is not surprising that the mechanisms that recognize damage are complex and as yet only partially understood. See Figure 3.6.
Fig. 3.6 Initiation of acute inflammation. Acute inflammation can be induced by sterile trauma or infection. Sterile trauma involving damage to blood vessels activates blood platelets and the coagulation cascade. It may also damage mast cells. All of these can release mediators (some examples are shown) that induce the vascular changes typical of acute inflammation. In infections, alarm cells such as epithelia, macrophages and mast cells can recognize pathogens via pattern recognition receptors (PRRs) which stimulate the release of inflammatory mediators. The skin and gut are illustrated, but these principles apply to epithelial tissues in general.
3.3.2.1 Responses to Trauma
In the case of mechanical trauma there are several pathways involved in the recognition of tissue damage. For example, trauma induces mechanical damage in mast cells that reside in connective tissues. In turn, they degranulate, releasing histamine and other mediators of inflammation. Trauma in almost all cases also induces haemorrhage (bleeding into the tissues). This eventually results in the stimulation of the healing reaction (Section 3.3.6). Cells that have died by necrosis break down their plasma membranes and it is suggested that they may release molecules called damage-associated molecular patterns (DAMPs). DAMPs, which are discussed a little more in Chapter 4, are poorly understood, but may include a very wide range of molecules that signal to the immune system that cellular or tissue damage has occurred.
3.3.2.2 Responses to Infection
Mechanical trauma may lead to entry of pathogens, as for instance in an infected wound. However, many pathogens can breach the natural barriers in the absence of trauma and it is a necessary property of many pathogens that they can do this (e.g. following inhalation or ingestion). For example, influenza virus can infect and damage the epithelia lining the airways, despite the presence of mucus. In fact this often leads to secondary infection of the damaged tissues by bacteria since these barriers have now been disrupted. Similarly Salmonella can evade stomach acid, bile and mucus to invade the intestinal epithelium (in contrast to the commensal bacteria which do not invade). On the other hand, some viruses and other pathogens can be injected directly into the bloodstream through the bite of an insect or mammal, such as those that cause malaria, plague and rabies. In addition, the larval forms of schistosomes can even burrow through the skin from infected water and ultimately spread through the bloodstream to infect other organs such as the liver (see Chapter 2).
Given the ability of pathogens to evade or avoid barriers, you will understand that it is essential for mechanisms to be present in all parts of the body, in all tissues and organs, that are able to detect and respond to any breach in natural defences which represents danger and to signal alarm to other components of the immune system.
The recognition that infection has occurred in tissues requires the presence of cells that can sense that this has occurred. These cells include the epithelial cells lining anatomical barriers which are usually the first point of attack of a pathogen. Underlying these surfaces, and in essentially all tissues, are tissue-resident (or at least very long-lived) immune cells that may be specialized to sense danger. These particularly include resident macrophages and mast cells. The general feature shared by these cells in peripheral tissues is that they have receptors called pattern recognition receptors (PRRs) that are able to recognize molecules, which are present only in or on pathogens, called pathogen-associated molecular patterns (PAMPs) (Section 1.2.3.1). PAMPs act as agonists for different types of PRRs and stimulate resident cells to secrete molecules that act on blood vessels to cause local inflammation (inflammatory mediators).
Crucially in early defence, resident macrophages can rapidly detect the presence of pathogens and quickly initiate inflammatory responses and innate immunity. For example they can be triggered to synthesize and secrete pro-inflammatory cytokines such as interleukin (IL)-1, IL-6 and tumour necrosis factor (TNF)-α. Mast cells are also present in all loose connective tissues such as those underlying mucosal epithelia. They contain cytokines and other pre-formed inflammatory mediators in their granules. Following stimulation (e.g. by trauma, as above, as well as infection) they release these mediators, and synthesize and secrete others that contribute to the inflammatory response. These responses are discussed in more detail in Chapter 4.
For now, the important principle to understand is that, after infection, the dynamic anatomy of innate responses involves induced changes in the structure and function of peripheral tissues at the local site (local inflammatory responses) so that effector cells and molecules can be recruited to eliminate that infection. Other changes, in more distant tissues (systemic inflammatory responses) are also needed, so that the availability of these cells and molecules can be increased.
3.3.3 Local Inflammatory Responses
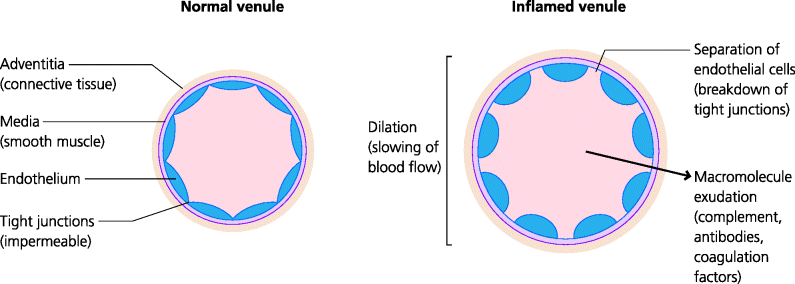
The effector cells and molecules that are needed to mount effective innate immunity are not normally present in tissues; they cannot cross non-inflamed endothelium and are restricted to the blood. Blood is primarily a distribution system, and in defence its function is to send cells and molecules to sites where they can mediate their effects. How can cells and molecules be directed to the sites where they are needed? Arterioles are involved – nervous impulses from the area of inflammation trigger an axon reflex that leads to arteriolar dilatation, and the increased blood flow delivers more leukocytes to the inflamed area. However, in acute inflammation, changes in the endothelial cells of specialized blood vessels, called post-capillary venules, are central and crucial. Post-capillary venules are thin-walled vessels lacking the layers of muscle and connective tissue found in larger vessels. Hence, it is easier for things to get across them, from the blood into the tissues, but to do so the endothelial cells that line them first need to be changed or activated. Endothelial cells in venules can be activated by a variety of mediators (discussed in more detail in Chapter 4). Venule dilatation causes slowing of blood flow, giving leukocytes increased opportunity to interact with the endothelium. Increases in permeability permit egress of macromolecules while changes in adhesion molecule expression permit leukocyte binding and recruitment. See Figure 3.7.
Fig. 3.7 Vascular changes in acute inflammation. Inflammatory mediators such as histamine act on venule smooth muscle to cause dilatation, leading to slowing of blood flow. Mediators also act on endothelial cells, leading to the breakdown of tight junctions – forming gaps that permit the exudation of blood plasma containing macromolecules such as antibodies, complement and coagulation factors. Changes in adhesion molecule expression also lead to leukocyte recruitment (see Figure 3.8).
3.3.3.1 Recruitment of Soluble Effector Molecules
A major alteration in the physiology of infected tissues is that the permeability of venules in the infected area is increased. Molecules such as TNF-α (secreted by macrophages and mast cells) and histamine (released from mast cells) stimulate the breakdown of tight junctions between endothelial cells, causing the formation of physical gaps. The increased permeability permits large amounts of plasma to enter the tissues, causing the characteristic swelling (oedema) of acute inflammation. The plasma contains proteins normally confined to the blood and these include large molecules such as complement components and (usually much later) antibodies which can now enter the extravascular space. Two main properties of these molecules are to further stimulate acute inflammation, mainly via the actions of breakdown components of activated complement proteins on mast cells, and to act as opsonins, coating pathogens to facilitate their uptake by the neutrophils and additional macrophages that are recruited to these sites (below).
3.3.3.2 Recruitment of Cellular Effectors
The increased permeability seen in acute inflammation is not, however, sufficient to target leukocytes to the inflamed area. The only way cells can identify the site where they need to go is via molecules expressed on endothelium. Hence, changes in molecules expressed on the endothelium of venules permit the migration of blood leukocytes into extravascular tissues. (Molecules secreted into the blood would be of no use – they would just be washed away.) Inflammation changes the adhesiveness of endothelial cells for leukocytes in selective ways, ensuring that migration is regulated.
Leukocyte–Endothelium Interactions
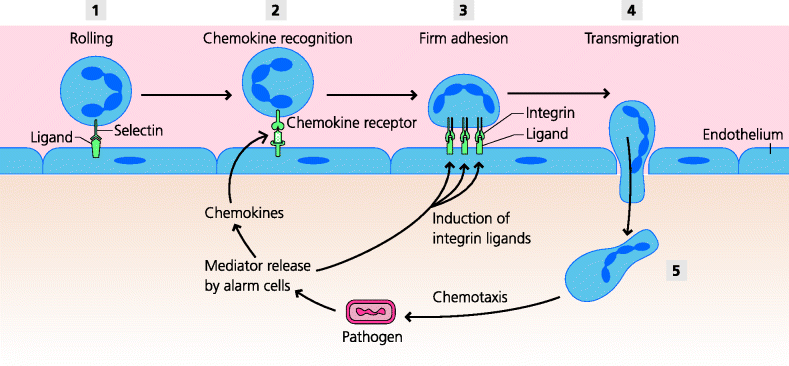
The first stage in leukocyte recruitment requires that the appropriate cells are targeted to the inflamed tissue. (Remember that each leukocyte has its own specialized functions and that, in general, these can only deal effectively with certain types of infection; Section 1.4.2). Three particularly important and distinct molecular recognition systems interact to direct specific types of circulating leucocytes to the appropriate tissue. Selectins on leukocytes bind carbohydrate ligands on endothelial cells to permit loose adhesion to the endothelium and vice versa. This loose adhesion then permits the leukocytes to bind to chemokines that are themselves bound to heavily glycosylated molecules on endothelial cells. This binding subsequently stimulates tight adhesion mediated via integrins. There are different selectins and integrins, and many different chemokines and chemokine receptors. Hence, different permutations and ombinations of these molecules permit the delivery of different leukocytes to different sites in a highly selective manner. The crucial outcome of this system is that it permits the recruitment of host defence cells to sites of infection quickly and appropriately – to the right place at the right time. Thus, in acute inflammation, neutrophils and monocytes are delivered. In chronic inflammation, monocytes and (later) activated lymphocytes are delivered. In parasitic and allergic responses, eosinophils and/or basophils arrive at the appropriate sites. In other words, these molecules act as an address for leukocytes and the complementary molecules on endothelial cells are in fact sometimes called vascular addressins. This can be described as a post code system, or ZIP code system if you happen to live in the United States. See Figure 3.8 and Box 3.1.
Fig. 3.8 Leukocyte recruitment in acute inflammation. (1) Leukocytes, particularly neutrophils, use selectins to form loose adhesions to carbohydrate ligands on venule endothelium and they roll along the endothelium. (2) This permits them to interact with chemokines bound to endothelial surface molecules. (3) The chemokines stimulate neutrophil integrins to increase their affinity, permitting strong adhesion to their ligands on the endothelium. (4) Neutrophils migrate to junctions between endothelial cells and cross the endothelium into extravascular tissues. (5) Chemotactic factors then draw the neutrophils to the site of infection. The underlying alarm cells are responsible for producing the chemokines and inducing the endothelial ligands (vascular addressins) in this process.
The principle of selective delivery of leucocytes to appropriate sites is absolutely crucial to understanding defence against infection, and is also crucial for thinking about potential therapies in non-infectious situations such as autoimmune diseases, transplant reactions and cancer (Chapter 7). Thus, modulation of leukocyte migration is being tested in clinical trials for treatment of autoimmune diseases and could possibly be used to prevent transplant rejection or even to stimulate tumour destruction.
Leukocyte Function in Inflammation
Virtually all tissues and organs can be stimulated by infection to generate signals that may act locally to trigger secretion of defence molecules. For example most epithelial cells can be stimulated to secrete defensins, which have antimicrobial activity (see Chapter 4). Importantly, infection can also stimulate the recruitment of cells and molecules (above) that cooperate to halt, or at least slow down, the development of the infection.
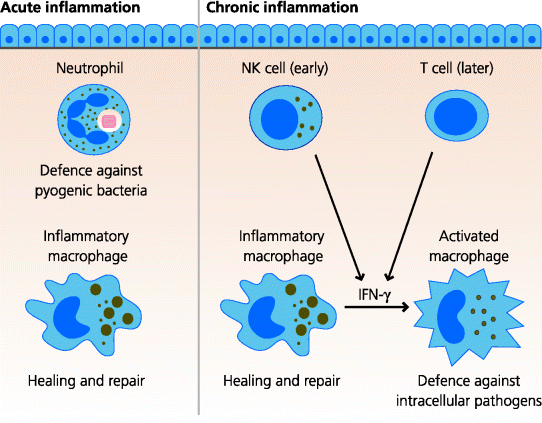
In acute inflammation stimulated by infection the major, initial, cellular effect is the recruitment of neutrophils into the site of infection. These cells are normally present in large numbers in the bloodstream and, once they have been recruited to inflammatory sites, they can efficiently phagocytose and kill some bacteria before dying. Stored neutrophils can also be released rapidly from bone marrow (below). Partly because of their rapid mobilization, neutrophils are particularly important in defence against pyogenic (extracellular) bacteria, which divide rapidly in extracellular sites, and some fungi (see Chapters 2 and 4). A little later, but in smaller numbers, blood monocytes are recruited into the acutely inflamed tissues where they differentiate into macrophages with additional, specialized functions compared to the resident cells that helped to trigger the initial inflammatory response. Their main function in pyogenic infection, however, is probably to regulate healing, repair and tissue remodelling. In other infections, such as those with mycobacteria, which induce chronic inflammation, recruited macrophages can be activated to become potent anti-microbial cells. These classically activated macrophages are able to kill intracellular bacteria such as Mycobacterium tuberculosis, but are also capable of causing much tissue damage because of their excessive secretory activity. See Figure 3.9.
Fig. 3.9 Leukocyte function in inflammation. In acute inflammation caused by pyogenic bacteria, neutrophils are the most important bactericidal cells. Recruited (inflammatory) macrophages are important in removing the damaged tissues and regulating the healing response. In chronic inflammation, recruited (activated) macrophages are crucial for killing intracellular bacteria such as mycobacteria following their activation interferon (IFN)-γ from NK cells or T cells. Macrophages also both cause tissue damage and are responsible for regulating healing and repair.
3.3.4 Effects of Inflammatory Mediators on Distant Tissues: The Acute-Phase Response and Systemic Inflammatory Responses
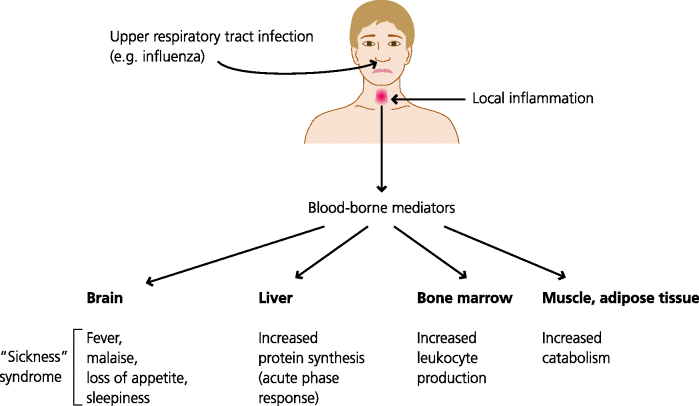
The cytokines produced by tissue-resident macrophages and mast cells in response to infection do not always just act locally. If they are produced in large enough amounts they have effects on anatomically distant sites. For example, TNF-α can signal to the bone marrow to increase the output of neutrophils, IL-1 can signal to the hypothalamus to increase temperature (fever), and IL-6 can signal to the liver to induce the synthesis of a large number of defence molecules at high levels, including opsonins and other agents. The latter is called the acute-phase response. See Figure 3.10.
Fig. 3.10 Systemic effects of inflammation. Cells in inflamed tissues release pro-inflammatory mediators into the blood. These can act on distant organs such as the brain to cause fever, malaise and loss of appetite, and on the liver to increase synthesis of defence-related proteins such as complement components. They also act on the bone marrow to cause release of stored leukocytes and to increase the production of leukocytes from stem cells. They act on muscle and adipose tissue to increase catabolism, generating energy. The latter is crucial; to increase body temperature by only 1 °C requires as much energy as an adult walking 35–40 km.
3.3.5 Initiation of Adaptive Immunity
The final important principle to emphasize is that many of the changes that occur in inflammation are absolutely crucial to the initiation and regulation of adaptive immune responses against infectious agents. The ways in which T cells differentiate after activation is very much determined by signals generated by the innate immune system. In fact, generally speaking, it is likely that adaptive immune responses could not be triggered at all without innate responses occurring. This topic is discussed in a little more detail in Section 3.4.
3.3.6 Regulation of Inflammation, Healing and Repair
3.3.6.1 Regulation of Inflammation
What is it that stops the inflammatory process? Of primary importance, of course, is the removal of the agent that initiated the inflammation. We see the importance this in situations where the agent cannot be removed: it may be resistant microbes such as Mycobacteria, indestructible particles such as silica or a self component as in autoimmune disease (Chapter 7). In all these cases the inflammation is prolonged, becoming chronic. Cessation of inflammation however, is also an active process. Lipid mediators such as lipoxins and resolvins inhibit the recruitment and activation of neutrophils, and may direct macrophages to areas of cell death, enabling them to clear the debris. Cytokines such as IL-10 may later modulate the functions of macrophages, for example by stimulating them to secrete transforming growth factor (TGF)-β that promotes healing.
3.3.6.2 Healing and Repair in Acute Inflammation
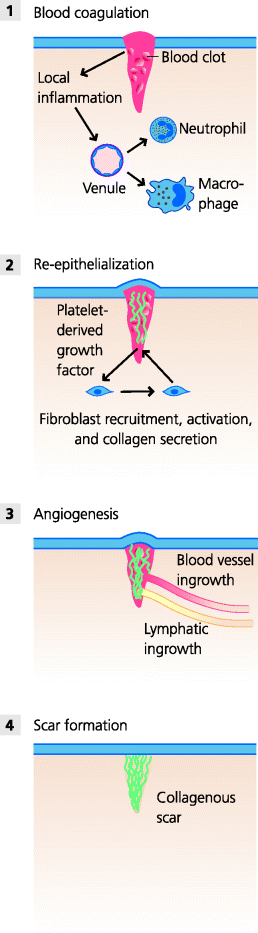
Acute inflammation inevitably causes some tissue damage, and it is a crucial part of all inflammation that it stimulates the processes of healing and repair. In the simplest forms of damage, say a cut with a sterile scalpel during a surgical procedure, blood platelets adhere to the edges of the cut vessels, forming a platelet plug. Fibrin formed by blood coagulation stabilizes this plug and forms the first layer of repair. Blood platelets degranulate as they adhere, releasing factors such as platelet-derived growth factor (PDGF), which are crucial for later stages of repair. These and other factors cause changes in the expression of adhesion molecules by local venules, causing monocytes to adhere and cross into the inflamed area. See Figure 3.11.
Fig. 3.11 Healing of a sterile wound. (1) A blood clot forms at the site of the wound. Local inflammation stimulates the activity of macrophages to release growth factors for fibroblasts, as do activated platelets in the clot. (2) These factors induce the recruitment and division of the fibroblasts and stimulate them to make collagen and other connective tissue molecules. (3) Other factors stimulate the in-growth of blood and lymphatic vessels (angiogenesis). (4) Epithelial cells divide to cover the wound and eventually the site of the wound is represented by a small, collagenous scar.
The recruited monocytes can now differentiate into inflammatory” macrophages See Chapter 1. These macrophages have crucial functions in repair including the following:
At the same time other factors stimulate the in-growth of blood and lymphatic vessels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


