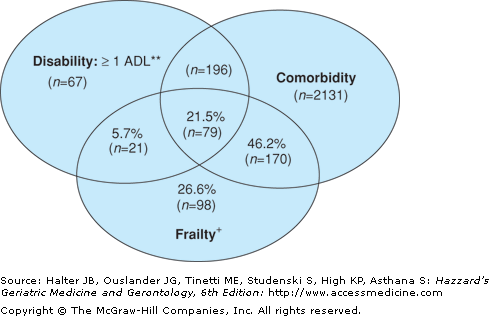Frailty Is at the Core of Geriatric Medicine
The cornerstone, even raison d’etre, of geriatric medicine concerns the identification, evaluation, and treatment of frail older adults and prevention of loss of independence and other outcomes for which they are at risk. The proportion of frail within the older population is high and will increase with the aging of society. A focus on frailty has been a consistent theme in geriatric theory and practice. In 1990, Fretwell stated “frailty in an individual (is) defined as an inherent vulnerability to challenge from the environment.” Because of the high-risk status of frail older adults, geriatric medicine seeks to intervene in frail patients to prevent or minimize illness and dependency. In 1992, a conference on the physiologic basis of frailty agreed that controversy on definition and limited understanding of etiology hindered preventive strategies. In 1993, W. Bortz stated that “A major threat to active life expectancy is the development of frailty…. Despite absence of easy categorization, there is no question as to the immense participation of frailty in both individual and composite morbidity and mortality. Recognizing this pervasive impact on well-being, it is strange to note the lack of critical insight that attends it.” Baltes and Smith observed that the oldest old, those in the “fourth age” after 85 years (in developed countries), are particularly biologically vulnerable and frail and have compromised ability to tolerate stressors. As a result, their well-being is increasingly dependent on the use of extrinsic compensations to maintain life and autonomy, because there is such diminished ability to compensate physiologically. These observations frame the conceptual understanding that aging is associated with increased likelihood of frailty, and that older persons have reduced physiological reserve than younger persons and these changes are likely independent of disease. Over the past 15 to 20 years since the statements above, we have attained increasing clarity about the definition and characteristics of frailty and its import and etiology, and a new basis for prevention and interventions. This chapter seeks to synthesize this knowledge.
Geriatric medicine has found the concept of frailty compelling for several reasons (Table 52-1). First, frail individuals are perceived to constitute those older adults at highest risk for a number of adverse health outcomes, including disability, dependency, institutionalization, falls, injuries, acute illness, hospitalizations, slow or incomplete recovery from illness and/or hospitalization, and mortality.
|
Additionally, they have compromised ability to tolerate hospitalization or invasive procedures and are at high risk of related complications.
Second, frail older adults are thought to be a subset in high need of health care and community and informal support services, as well as long-term care. These special needs were the main basis for the development of comprehensive geriatric assessment and creation of specific geriatric systems for care delivery as optimal clinical approaches to decreasing preventable adverse outcomes for frail older adults. The provision of care for this increasing subgroup of frail individuals is a critical clinical and public health concern. Although some decry the increased costs anticipated as the numbers of frail older adults increase, others, particularly in geriatric medicine, propose that redesign of health care delivery to optimize outcomes in these vulnerable older adults will lead to a health care system specifically tailored to chronic care delivery and, if so, less costly. The ability of the health care system to care effectively for those who are frail, and even to prevent frailty, will depend on our ability to grow a geriatrically expert, adequately sized clinical work force, and to provide appropriate economic incentives, recognizing that caring for frail older people requires exceptional skills and is extremely time-consuming.
Third, the prevalence of frailty is high, with estimates ranging from 10% to 25% of persons aged 65 years and older, with as many as 30% to 45% of those aged 85 years and older identified as frail. Such estimates are based on clinical perceptions of a notable change in vulnerability, health status, and clinical appearance with age in a substantial subset of older adults that is not explained by disease alone. These changes are often reported by family and friends of patients, as well as clinicians, when they describe the patient as “appearing” frail. This condition includes a composite image of loss of muscle mass, weakness, slowed pace of movement, decreased activity and engagement, and possibly unexplained weight loss—often in combination. This clinical characterization is supported by an extensive literature summarized by Ferrucci et al. (Table 52-2).
REFERENCE | MOBILITY | STRENGTH | BALANCE | MOTOR PROCESSING | COGNITION | NUTRITION | ENDURANCE | PHYSICAL ACTIVITY |
|---|---|---|---|---|---|---|---|---|
Winograd CH et al. | X | X | X | X | ||||
Ory MG et al. | X | X | X | X | X | |||
Pendergast DR et al. | X | X | X | X | ||||
Rockwood K et al. | X | X | ||||||
Tinetti ME et al. | X | X | ||||||
Gill TM et al. | X | X | ||||||
Campbell AJ et al. | X | X | X | X | X | X | ||
Dayhoff NE et al. | X | X | ||||||
Strawbridge WJ et al. | X | X | X | |||||
Chin APMJ et al. | X | X | ||||||
Vellas B et al. | X | X | X | X | ||||
Brown M et al. | X | X | X | X | ||||
Fried LP et al. | X | X | X | X | X | |||
Saliba D et al. | X |
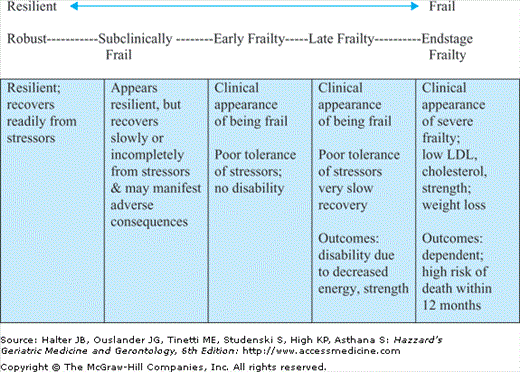
Fourth, it is thought that the increased risk of adverse outcomes associated with frailty is a result of an increased vulnerability to stressors itself caused by a decreased ability to maintain homeostasis when the individual is stressed. Stressors can be intrinsic, such as infection, or extrinsic, such as change in environment. There is some evidence to suggest that, in addition to those who already appear frail clinically, a subset of older individuals have subclinical frailty, i.e., have increased vulnerability to adverse outcomes in the face of stressors but without the clinical stigmata of frailty or any of its outcomes. Further, some clinical reports indicate that there is a subset of older adults with advanced frailty stigmata and significant outcomes, particularly disability or dependency, who have lost reserves and resilience to a point that they have a very high likelihood of dying within 6 to 12 months, and are quite unlikely to respond to therapies, including rehabilitative therapies. These differentiations of vulnerability—with or without the clinical appearance of frailty and its sequelae—are consistent with the idea of a continuum of frailty among older adults as a core component underlying the heterogeneity of health status observed with increasing age (Figure 52-1).
This continuum of frailty incorporates what is thought clinically to be a distinct causal pathway to disability, with frailty being a major etiologic risk factor independent of disease (Figure 52-2).
Note that the causal pathway from frailty to disability has not been considered in the conceptualization of the causal pathway to disability proposed by the World Health Organization and the Institute of Medicine, which map the effect of specific diseases and impairments. In contrast, conceptually, a survey in six U.S. academic centers and one in Great Britain indicated that 98% of geriatricians (both faculty and postdoctoral fellows) thought that frailty and disability were separate, but causally related, conditions; 90% of the geriatricians interviewed thought that frailty caused disability (of which 40% thought that this was usually or always the case), while 88% thought that disability caused frailty (of which 13% thought that this was usually or always the case) (Table 52-3). These close and bidirectional relationships likely cause frequent co-occurrence of frailty and disability and the conceptual confusion of the two.
RESPONSE | |||
|---|---|---|---|
Yes (%) | |||
QUESTION | No (%) | Sometimes | Usually/Always |
Are frailty and disability the same? | 97.5 | 2.5 | — |
Is disability a cause of frailty? | 12.5 | 75.0 | 12.5 |
Is frailty a cause of disability? | 10.0 | 50.0 | 40.0 |
Increasingly, frailty is thought distinguishable from disability (as an outcome) and comorbidity, although there are overlapping coprevalences (Figure 52-3).
Figure 52-3.
Venn diagram displaying extent of overlap of frailty with comorbidity (≥2 diseases) and ADL disability in people 65 and older participating in the Cardiovascular Health Study.
Total represented 2762 subjects who had comorbidity and/or disability and/or frailty. N of each subgroup indicated in parentheses. + Frail: overall n = 368 frail subjects (both cohorts). *Comorbidity: overall n = 2576 with 2 or more out of the following 9 diseases: myocardial infarction, angina, congestive heart failure, claudication, arthritis, cancer, diabetes, hypertension, COPD. Of these, 249 were also frail. **Disabled: overall n = 363 with an ADL disability: of these 100 were frail. From Fried LP, et al: Frailty in older adults: Evidence for a phenotype. From Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 56;M146–M156, 2001.
A fifth reason that the concept of frailty has had saliency for geriatricians is the mounting evidence that there is a decrease with age in the ability of disease alone to explain the increased variation in health status, outcomes, or response to therapy. Measures of subclinical organ system changes and physical functional and cognitive variables, rather than presence or absence of diseases, are the most powerful predictors of longevity and functional outcomes. For example, in the Cardiovascular Health Study, physiological and functional measures, rather than diseases, were significant predictors of 5-year mortality, with the exception of congestive heart failure. In analyses from the Canadian Study of Health and Aging and the Gothenburg H-70 Cohort Study, Rockwood et al. demonstrated that deficit accumulation, from a list of 40 to 51 symptoms, signs, and disabilities as well as diseases, predicts mortality in a linear fashion in relation to a baseline count of deficits, with all deficits weighted equally. In the InCHIANTI study, slow walking speed (<0.8 m/s) was as strong a predictor of mortality as a diagnosis of malignant cancer. Thus, nosologically defined diseases, alone or in combination, are not sufficient to explain functional outcomes in older persons, suggesting that a disease-independent, age-related alteration accounts for the progressively higher variability in health, impairments, prognosis, and outcomes with aging. This condition, if not equivalent to frailty as defined above, is certainly reminiscent of this condition. Age-related frailty may explain why older age is associated with the increased variability in response to treatments, both in terms of effectiveness and risk of side effects, not explained fully by disease status. The increased risk of iatrogenesis is likely a product of the altered reserves and associated physiologic vulnerabilities that are components of frailty.
Sixth, with increasing age, there is a concurrent, increased susceptibility to multiple chronic diseases that is not explained by “classic” risk factors. This increased susceptibility with no evident pathogenetic connections in risk between the multiple diseases could be related to the progressive collapse of the regulatory network of biological signals aimed at maintaining the homeostatic equilibrium. For example, there is evidence that old age is associated with a low grade, chronic proinflammatory state, which also appears to be causally related to frailty (see further). Physiologic dysregulation with aging in this and many other systems may lead both to aggregate loss of reserves and vulnerability and to disease-specific manifestations such as atherosclerosis. Such associations could lead to correlation between frailty and disease, which does not indicate a true causal association. In some instances, frailty maybe the terminal stage of selected chronic diseases. Overall, as represented by Figure 52-3 and Table 52-3, frailty, comorbid disease, and disability are overlapping, causally related, but distinct entities.
Seventh, given the discussion above, it would appear that frailty is a condition of impending deterioration in health and functional status that requires immediate attention to prevent disability and other associated outcomes. It requires substantial clinical expertise to both recognize those who are frail and/or vulnerable, and accurately diagnose and effectively intervene to prevent adverse outcomes or frailty itself. Because of the complexity of presentation, attendant vulnerabilities, and multiple health problems likely to be concurrently present, health care in frail older people needs to be skilled, intensive, and continuous to be effective and, therefore, is intrinsically more expensive. Current U.S. health care models are driven, however, by a unique focus on disease diagnosis and a primary organization for acute, event-driven care, with reimbursement for care heavily driven by specific diagnostic categories. This approach does not permit addressing effectively the complexity of care issues, nor the specialized care required when frailty is also present, or for frailty in the absence of disease. In regards to the former, for example, care for a patient with a hip fracture is erroneously considered the same, regardless of age or frailty status of the individual, with reimbursement the same as well. However, expert geriatric care for a frail older patient with a hip fracture has a greater real cost of caring and likely commensurate benefit. Redesign of care and reimbursement to match the needs of such patients will likely lead to improved care outcomes and potentially decreased overall costs. Overall, postulating the existence of age-related frailty addresses and incorporates all of the issues above, assuming that frailty can be operationalized and recognized in clinical practice.
What Is Frailty?
There is strong consensus among geriatricians and gerontologists that frailty is a clinical state of increased vulnerability and decreased ability to maintain homeostasis that is age-related and centrally characterized by declines in functional reserves across multiple physiologic systems. This vulnerability is age-related and also related to, but distinct from, disability and disease states (see Table 52-3 and Figures 52-2 and 52-3). There is general agreement as well that frailty results from underlying physiologic and/or biologic alterations that are age-associated and maybe compounded by single or multiple diseases, or even be an end-stage outcome of severe disease. Key systems thought to be involved in development of frailty include musculoskeletal, hormonal, immune, and inflammatory systems, with likely contributions from the autonomic and central nervous systems. Sarcopenia, or loss of muscle mass with aging, is thought to be a central manifestation of frailty. In fact, a change in body composition with a progressive decline in lean body mass, mostly represented by muscle, is an almost obligatory manifestation of aging and, past a threshold severity, of frailty itself. However, the rate of age-associated decline in muscle strength and muscle mass is profoundly modulated by a number of physiologic factors including inflammation, hormones, neurological integrity, nutritional status and physical activity, along with other contributors. It is noteworthy that the multiple physiologic systems that affect sarcopenia are also thought to contribute to generalized dysfunction that is aging-related, an issue well beyond sarcopenia itself. This is consistent with the theory proposing that it is the aggregate dysregulation of many systems that results in the vulnerability and clinical presentation of frailty more than the dysregulation of any one system.
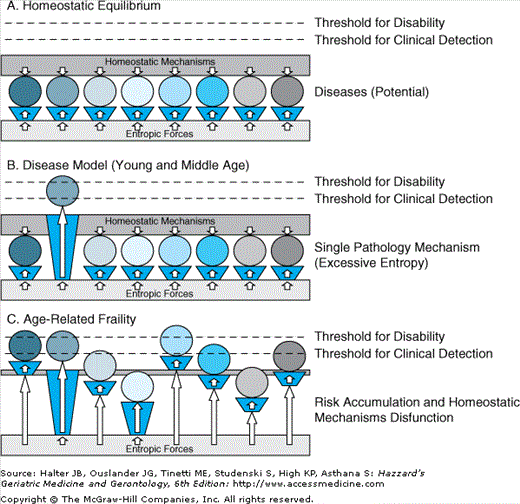
One way to understand frailty is by comparison with the traditional concept of disease. Traditionally, diseases are defined by symptoms, signs, and pathophysiologic mechanisms. Specific diseases impair selected aspects of the homeostatic equilibrium that is essential for life. A large variety of stressors continuously challenge the homeostatic equilibrium and facilitate the emergence of diseases. Health is characterized by a perfect equilibrium between stressors and homeostatic mechanisms (Figure 52-4A). Diseases emerge when specific physiological system(s) or anatomical structure(s) are impaired and pose an entropic challenge that cannot be fully counteracted by homeostatic mechanisms. This is typical of young and middle age, where diseases are stochastic, somewhat “rare” events that often target a specific mechanism (Figure 52-4B). For example, a brain hemorrhage is unlikely to occur in a healthy, young individual unless there is clear cause (malignant hypertension, aneurysms etc). In old age, this can more easily result from a combination of multiple processes that include both strong entropic challenges and thinning of the mechanisms that maintain the homeostatic equilibrium (Figure 52-4C). Under the assumption that homeostatic mechanisms are general, it is more difficult with aging to recognize a specific pathophysiological mechanism for each disease and clinical presentation maybe atypical. As a result, treatment of specific diseases one by one is less likely to be successful. Understanding the causes of derangement of the homeostatic mechanisms and possibly correcting them is more likely to be beneficial than targeted treatment or a specific system. According to this interpretation, prevention in old age may need to be focused on reinforcing homeostatic mechanisms rather on risk factors for specific diseases. This is compatible with the idea that change in nutrition and exercise are the only interventions that have been shown to prevent disability.
Figure 52-4.
Diseases in young and old age. Homeostatic equilibrium is maintained by robust function and interconnections between multiple physiologic systems (A). When one system is dysregulated or impaired, as in a specific disease, disability can result in the specific areas of function affected by the disease (B). The derangement of general homeostatic mechanisms characteristic of frailty induces a multiple systems impairment which emerges clinically as frailty as well as development of multiple diseases and complex patterns of disability (C).
There are several dominant theories as to the underlying causes of physiologic vulnerability and compromised homeostasis of frailty. They include the following:
Frailty comes from accumulation of potentially unrelated diseases, subclinical dysfunctions, and disability across organs, parts, and systems of the body. This approach has been posited by Rockwood and colleagues, who have operationalized this theory in terms of a summary of all potential deficits present in an individual (symptoms, signs, diseases, geriatric conditions, laboratory abnormalities, disabilities); a simple count of all such deficits assessed has been shown to predict mortality. This approach indicates that a summary measure of deficit accumulation across many different types of health conditions at many levels (functional, clinical, physiological) predicts risk of mortality. Inferentially, frailty is an intermediary, almost latent, construct that is the summary effect of all of these deficits on homeostatic reserves; the number of deficits leads to a dose–response relationship with mortality, presumably through this intermediary mechanism.
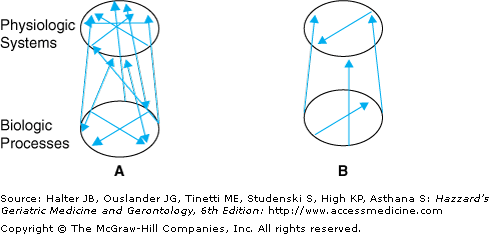
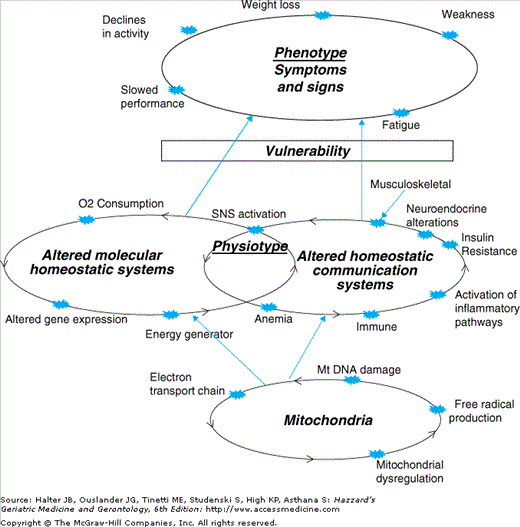
Frailty is a unique pathophysiological process: This theory posits that frailty can be characterized as a primary defect, which involves the diminution of physiologic function and, eventually, breakdown of homeostatic mechanisms. This could result from alterations in a range of basic biological mechanisms, which then lead to dysregulation of multiple physiologic systems. These systems are known to mutually affect each other, providing a rich network of homeostatic regulation and ability to compensate, to a degree, if any one system is impaired. This redundant network, with intact function within and between systems, underlies reserves and resiliency to stressors (Figure 52-5A). Dysregulation of multiple systems with aging, and decreased effectiveness of interconnections (Figure 52-5B) could lead to depletion of reserves and compromised ability to maintain homeostasis in the face of stressors. Ultimately, this could lead to a negative spiral of declining function. Basic biological systems involved may well include those that maintain a stable production, distribution, and utilization of energy, while key physiologic systems include hormones, immune, inflammatory, and neurological processes. Decreased energy available would diffusely affect multiple physiological systems, leading to compromised function both within and between systems. Further, decreased availability of energy could underlie decline in physical function, especially in tasks requiring endurance (Figure 52-6). A similar scenario could occur as a result of other basic biological alterations with aging, such as shortened telomeres or excessive free radical damage. Alternatively, or additionally, frailty may result from a progressive loss of complexity in the function of individual physiologic systems and in their regulation of homeostatic responses, leading to both chronic over- or underfunction.
Figure 52-5.
Conceptual summary of interconnections between biologic and physiologic systems that comprise a stable homeostatic system, with rich network that offers redundancy and reserves. (A) Robust interconnections between these systems, as indicated by multiple arrows. (B) Many fewer arrows, signifying decreased function of different systems and weakened interconnections, threatening homeostasis. From Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005(31):pe24, 2005.