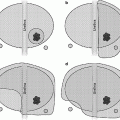
Fig. 10.1
Perineal template with inserted cryoprobes to treat right side distal lesion
Fig. 10.2
Inserted cryoprobe needles as seen on ultrasound image
Fig. 10.3
Iceball—transverse view
Fig. 10.4
Iceball—sagittal view. Notice sparing of proximal prostate
Current Studies: Oncologic and Functional Outcomes
Cryotherapy is the most studied ablative therapy. Onik et al. were first to report outcomes of FT in 2002 followed by an update of their experience in 2008. They reported on 48 patients with a mean follow-up of 54 months (range 2–10 years). Ninety-four percent had no evidence of cancer according to ASTRO (American Society of Therapeutic Radiology and Oncology) criteria. Potency was maintained in 36 of 40 patients (90 %) and all were continent after treatment [20].
Ellis et al. reported on 60 patients treated with focal cryotherapy. The mean follow-up was 15 months. 84 % of the patients were biochemically disease-free (ASTRO criteria) and 3.6 % reported urinary incontinence [21].
In 2007, Lambert et al. reported on 25 men treated with hemiablation of the gland. Mean follow-up was 28 months. Eighty-four percent had experienced no biochemical failure, defined as 50 % PSA increase over nadir level. Seven patients underwent a repeat biopsy. One patient had prostate cancer in the area of previous cryoablation and 2 patients in the contralateral gland [18].
Bahn et al. reported on 31 patients. Biochemical disease-free rate by ASTRO criteria was 92.8 %. Biopsy in one patient with biochemical recurrence demonstrated cancer at the apex of the untreated side. Potency preservation rate was 88.9 % (40.7 % with PDE-5 inhibitors) [22].
More recently Ward et al. published an update from the Cryo On Line Database (COLD) registry; biochemical disease-free rate was 75.7 % (ASTRO criteria) at 36 months, urinary continence was 98.4 %, and preservation of spontaneous erections 58.1 % [23].
Overall, biochemical disease-free rate is 75–94 % [18, 20–23]. Nevertheless definitions of biochemical recurrence and patient’s selection criteria were variable between studies. These studies are limited due to small number of patients and short follow up. The reported functional outcomes are encouraging, with a good potency and urinary continence rates. No other significant morbidities were reported (Table 10.1).
Table 10.1
Summarizes oncologic and functional outcomes of focal cryotherapy
Name | N | Mean follow-up (months) | BR criteria | BDF (%) | Potency (%) | Continence (%) |
|---|---|---|---|---|---|---|
Onik [20] | 48 | 54 | ASTRO | 94 | 90 | 100 |
Ellis [21] | 60 | 15 | ASTRO | 80.4 | – | 96.4 |
Lambert [18] | 25 | 28 | PSA nadir + 50 % | 84 | 71 | – |
Bahn [22] | 31 | 70 | ASTRO | 92.8 | 89 | – |
Ward [23] | 1,160 | 36 | ASTRO | 75.7 | 58.1 | 98.4 |
Future Direction
Focal Cryotherapy is a promising treatment option for selected patients with early prostate cancer. Future research should be directed towards establishing better means of characterizing clinically significant disease and developing improved image technologies to target treatment.
High-Intensity Focused Ultrasound
Overview
HIFU was first described in 1995 as a technique to treat localized prostate cancer. Most of the current reports describe whole prostate gland treatments. HIFU can also be used for focal tumor ablation with the goal of sparing normal gland and minimizing the adverse effects of whole gland treatment. There are currently two devices available for treatment: The Ablatherm HIFU device (EDAP S.A., Lyon, France) and The Sonable 500 (Focus surgery, IN, USA). Both devices are widely used in Europe, Canada, and Japan. HIFU is still considered investigational in the United States and is not currently approved by the Federal Drugs Administration (FDA). Several trials are currently in progress to establish the efficacy and safety of HIFU.
Mechanism of Action
During HIFU ultrasound waves are emitted from a transducer and absorbed in the target area inducing necrosis. Two main mechanisms are involved in the HIFU ablation effect: A thermal effect is heat generation due to absorption of the acoustic energy with a rapid elevation of temperature in the targeted tissues, which denatures proteins, destroys lipid-based membranes, and finally results in instantaneous and irreversible coagulative necrosis. This is the primary mechanism for tumor cell destruction. The mechanical effect leads to cavitation causing additional damage to the prostate and periprostatic tissue. The treatment area is heated for 3 s and cooled for 6 s using real-time images. Surrounding tissue is minimally affected as the energy decreases sharply outside the target zone [24–26].
Procedure
Ablatherm ® after induction of general anesthesia, the patient lies on his left side, thighs, and legs flexed 90° on the trunk. A transrectal HIFU probe is inserted per rectum. The probe delivers a beam of high-focused convergent ultrasounds, causing heat and tissue destruction. The ultrasound beam absorption creates an immediate increase in temperature (85–100 °C). The treatment is performed using contiguous HIFU shots 1.8 mm apart with 4-s shot duration and a 12-s interval between shots. At the end of the procedure an 18 F Foley catheter is placed for 1–2 weeks.
Sonablate ® 500 after induction of general anesthesia, the patient is placed in the lithotomy position and HIFU probe is introduced per rectum. Treatment is monitored with real-time TRUS. After the procedure, an 18 F Foley catheter is placed and left for 1–2 weeks.
Current Studies, Oncologic, and Functional Outcomes
In 2008, Muto et al. reported on 29 patients who underwent transrectal HIFU (Sonablate 500). Two years biochemical disease-free rates by ASTRO criteria in patients with low and intermediate risk prostate cancer were 83.3 % and 53.6 % respectively [25].
Ahmed et al. reported a prospective study phase I/II trial in 20 men with prostate cancer who underwent a transrectal hemiablation of the prostate with the Sonablate 500 device. Patients were divided into low (n = 5) and intermediate risk (n = 15). Follow-up included MRI; TRUS-guided biopsies and PSA measurement at 1 month after the procedure and every 3 months thereafter. There was no histological evidence of cancer in 89 % of treated lobe. A trifecta status (pad-free, leak-free continence, erections sufficient for intercourse and cancer control) was achieved in 89 % at 12 months [26]. Ahmed et al. reported their results on 41 men treated between 2007 and 2010; using the Sonable 500 device and who were diagnosed by a combination of multiparametric MRI and Transperineal Template Mapping Biopsies (TTMP). Follow-up was scheduled every 3 months after treatment. Questioners were used to assess potency and incontinence. Eighty-nine percent (31 of 35 patients) described erections sufficient for penetration at 12 months. Fourteen required phosphodiestrerase-5 inhibitors. Of 38 men with no urinary leak at baseline 100 % were leak-free by 9 months. Thirty-nine of 41 patients underwent postoperative biopsy. Nine (23 %) had evidence of cancer. MRI at 6 months showed residual cancer in the treated areas in nine men; seven of whom had cancer confirmed on biopsy. Of those with positive biopsies, four patients underwent retreatment and none showed significant disease at 12 months on MRI [27]. These studies demonstrate good morbidity outcomes and promising cancer control rates. Limitations to these studies included small number of patients and short-term follow-up. Some authors considered hemiablation as a focal therapy with no consensus on definition regardless of grade, volume, or location of the tumor. Hemiablation may represent overtreatment since low-volume and low-grade lesions may be treated with more focused therapy.
Future Direction
Additional studies and more conclusive findings are needed. Trials are currently ongoing (NCT01194648, NCT00988130, NCT00987675) to establish the safety and efficacy of HIFU.
Photodynamic Therapy
Overview
The first report describing PDT for prostate cancer with light-sensitive agent using a transurethral approach was published in 1990 [28]. PDT is an experimental ablative technology which employs photosensitizing properties selectively taken up by prostate cancer cells and produces free oxygen radicals upon exposure to light of a specific wavelength which results in the destruction of the tissue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree