Fibromyalgia and Myofascial Pain Syndromes: Introduction
Fibromyalgia and myofascial pain (MP) are among the most common musculoskeletal disorders from which older adults suffer. These disorders represent opposite ends of the pain spectrum with the discrete character of MP at one extreme and the widespread symptoms of fibromyalgia at the other. MP may be acute or chronic, and is associated with taut muscle bands and hypersensitive areas called trigger points. Fibromyalgia syndrome includes symptoms of sleep disruption, fatigue, and psychological distress in addition to widespread pain. Both fibromyalgia and MP syndromes may result in significant functional impairment and cause suffering and disability comparable to that of rheumatoid arthritis and osteoarthritis. Diagnosis of these disorders is grounded in appropriately targeted history and physical examination; these are the tools required to avoid unnecessary ordering of “diagnostic” tests and foster implementation of appropriate management strategies.
Fibromyalgia Syndrome
While a number of fibromyalgia classification criteria have been proposed, the criteria developed by the American College of Rheumatology are used most commonly. These criteria, which are 81% sensitive and 88% specific, allow fibromyalgia patients to be distinguished from patients with widespread pain caused by other rheumatological disorders (e.g., systemic lupus erythematosus, rheumatoid arthritis). They include a history of generalized body pain (i.e., pain in at least three of four body quadrants) for at least 3 months duration and at least 11 out of 18 specific tender points on physical examination. Although initially developed for classification of fibromyalgia, practitioners tend to regard them as required for diagnosis, although this is not accurate. Older adults who present with widespread pain and other supportive clinical features (see below) should be considered to have fibromyalgia even if they do not precisely fulfill the ACR criteria. These criteria are best used as a general guide and to allow for study enrollment, not for strict use in the office setting.
The incidence of fibromyalgia syndrome (the proportion of new cases or first ever episodes) is difficult to measure in part because symptoms seem to ebb and flow over time. According to five large population studies, approximately 10% of the population has widespread pain. Of those with widespread body pain, approximately 2% meet ACR diagnostic criteria for fibromyalgia. Women are four to seven times as likely to have fibromyalgia compared to men, with the greatest prevalence in those 60 to 79 years of age. Patients with fibromyalgia also have been estimated to have a two- to sevenfold greater risk of suffering from depression, anxiety, headache, irritable bowel syndrome, chronic fatigue syndrome, systemic lupus erythematosus, and rheumatoid arthritis compared to healthy individuals.
While recent studies have added to our understanding of the pathogenesis of fibromyalgia, the exact cause is still unknown. Most studies suggest that abnormal central nervous system pain processing, known as central sensitization, plays a key role in fibromyalgia pathogenesis. Abnormal peripheral pain processing, peripheral sensitization, also contributes to fibromyalgia pathogenesis. The cause of sensitization is not known, but a variety of neuroendocrine and biochemical abnormalities are believed to be involved.
Early studies of fibromyalgia patients failed to consistently show abnormalities in the peripheral tissues. However, reexamination of this issue has uncovered differences between muscle samples from fibromyalgia subjects and healthy controls. One difference is higher levels of nitric oxide in muscles of fibromyalgia patients that may result in increased cell death. Other abnormalities that have been identified in muscles of fibromyalgia patients as compared with healthy individuals include lower phosphorylation potential and oxidative capacity as evidenced by lower levels of muscle phosphocreatine and ATP, as well as increased substance P, DNA fragmentation, interleukin-1, and perfusion deficits (see Staud reference). While the exact meaning of these abnormalities is unclear, the findings suggest an underlying difference in muscle metabolism of fibromyalgia patients as compared to people without fibromyalgia. Further study is needed to establish a relationship between these findings and the pain and fatigue fibromyalgia patients report.
Abnormalities of peripheral and central pain processing are well established in fibromyalgia. In the periphery, tissue sensitization results from changes in primary nociceptive afferents, increased neuronal excitability, and enlarged neuronal receptive fields. Central sensitization involves neuroplasticity in the brain and spinal cord. “Windup,” a normal finding of increased pain sensations after repeated exposures to a painful stimulus, is an example of central sensitization. In studies of fibromyalgia patients, the “windup” response is exaggerated compared to controls. Staud and colleagues studied fibromyalgia patients and healthy controls after repeated exposure to heat stimuli. While both groups had higher pain ratings after repeated exposures to heat, the degree of windup and temporal summation was significantly greater in fibromyalgia subjects. In addition, the fibromyalgia subjects had more prolonged after sensations compared with control subjects. Peripheral and central sensitizations contribute to the exaggerated pain response of fibromyalgia patients.
Altered activity of the hypothalamic–pituitary–adrenal axis (HPA), and abnormal levels of adrenocortical trophic hormone (ACTH) and urinary cortisol have been demonstrated in patients with fibromyalgia. Evidence suggests that the HPA axis may be less resilient than normal in fibromyalgia patients and that this and other HPA axis abnormalities may underlie the impaired response to stress that many of these patients exhibit. The review by Crofford provides an expanded discussion of the neuroendocrine abnormalities in fibromyalgia syndrome.
While there are no serologic tests to assist practitioners with making a diagnosis of fibromyalgia, a number of biochemical abnormalities have been identified in the context of research studies. Russell and colleagues have identified lower levels of serum serotonin and norepinephrine in patients with fibromyalgia compared to controls. Low platelet serotonin levels have also been identified. While cerebrospinal fluid (CSF) serotonin has not been measured in patients with fibromyalgia, its precursor and metabolic products have been demonstrated to exist at significantly lower levels in the CSF of fibromyalgia patients compared to controls. Norepinephrine’s metabolite, methoxyhydroxyphenyglycol, and dopamine’s metabolite, homovanillic acid, are also reduced in fibromyalgia patients. Further discussion of the biochemical abnormalities found in fibromyalgia patients is found in the review by Mease.
Abnormal levels of nociceptive neurochemicals have also been found in patients with fibromyalgia. Several studies have shown that substance P, a neuropeptide involved in pain transmission, exists in significantly higher levels in the CSF of fibromyalgia patients compared to those without fibromyalgia (see the Russell reference). Nerve growth factor (NGF), which promotes production of substance P, also is elevated in the CSF of fibromyalgia patients.
Researchers have used functional magnetic resonance imaging (fMRI) to help understand fibromyalgia pathogenesis. Functional MRI measures regional blood flow in the central nervous system in response to various environmental stimuli and fMRI studies have demonstrated increased central nervous system activity that corresponds to fibromyalgia patients’ subjective pain reports. In response to stimuli, which do not cause pain in controls, fibromyalgia patients report high pain scores and have augmented regional cerebral blood flow on fMRI.
Mounting evidence points to fibromyalgia as a heritable disorder. This evidence includes familial aggregation of fibromyalgia as well as a reduced pain threshold in the first-degree female relatives of fibromyalgia patients, even in those without overt clinical symptoms. Gene polymorphisms in the serotonergic and dopaminergic systems and a higher prevalence of polymorphisms in the promoter region of the serotonin transporter gene (5HTT) in fibromyalgia patients as compared to healthy controls also have been identified.
Fibromyalgia patients often report that they feel pain “all over.” Pain diagrams, on which patients are asked to shade painful areas of a human figure, are helpful in making a diagnosis. For fibromyalgia patients, these diagrams show diffuse shading on the right and left sides of the body as well as above and below the waist. We observe that some fibromyalgia patients shade, circle, or put an X through the entire figure. Patients generally rate their pain as moderate to severe in intensity. Over time, pain will fluctuate in severity but typically does not resolve completely. The quality of the pain may be variably described as deep aching, mild tenderness, or sharp sensations. Symptoms are generally constant throughout the day but often are worse in the morning and the evening. Triggers, including stress, cold weather, illness, and unaccustomed exertion, will likely increase pain. In addition to pain, 75% of patients report stiffness and over 50% report a sensation of swelling. Low back pain and chronic whiplash are relatively common, affecting 20% to 30% of the patients with fibromyalgia.
Fibromyalgia patients are likely to report a wide variety of nonmusculoskeletal symptoms, most commonly fatigue and difficulty sleeping. Sixty percent of patients report psychological and neuropsychological symptoms including anxiety, mental distress, and cognitive dysfunction. Thirty percent of patients report current depression with over 50% of patients reporting a history of depression. Headaches are also common. Uncommon symptoms (<20% prevalence) include tinnitus, dizziness, vertigo, and Raynaud’s phenomenon. Fibromyalgia may also coexist in 20% of rheumatoid arthritis patients, 30% of patients with systemic lupus erythematosus, and 50% of those with Sjögren’s syndrome. Table 123-1 lists the symptoms and syndromes commonly associated with fibromyalgia.
Musculoskeletal |
Stiffness |
Sensation of joint swelling |
Nonmusculoskeletal |
Fatigue |
Difficulty sleeping |
Dysesthesias |
Paresthesias |
Depression |
Anxiety |
Stress |
Dyspnea |
Palpitations |
Difficulty concentrating |
Tinnitus |
Dizziness |
Vertigo |
Associated Syndromes |
Headache |
Tension Type |
Cervicogenic |
Migraine |
Irritable bowel syndrome |
Pelvic pain |
Restless leg syndrome |
Interstitial cystitis |
Myofascial pain syndromes |
Associated Rheumatological Disorders |
Systemic lupus erythematosus |
Rheumatoid arthritis |
Sjogren’s syndrome |
Up to 80% of fibromyalgia patients report debilitating fatigue. This complaint encompasses mental fatigue and impaired concentration commonly referred to as “fibro fog,” physical fatigue after exertion, and general sleepiness. In fibromyalgia patients, these symptoms most often occur in the absence of other medical illnesses. Poor sleep quality seen in fibromyalgia patients is known as nonrestorative sleep. Patients awaken feeling unrefreshed even after a full night’s sleep. Other complaints include light sleep, frequent awakenings, and insomnia. As with other aspects of fibromyalgia, the cause of poor sleep and chronic fatigue is not fully known. Studies of sleep architecture in fibromyalgia patients have revealed abnormalities, which may account for daytime fatigue. One such finding is alpha wave intrusion into stage four sleep. In fibromyalgia patients, alpha waves, typically seen in stage one light sleep, are found in stage four slow wave deep sleep. In addition, fibromyalgia patients have a relative rapid eye movement (REM) sleep deficiency compared to healthy controls. These abnormalities, while not specific to fibromyalgia, may cause significant fatigue.
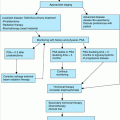
When evaluating the older adult with widespread chronic pain, the practitioner should be cognizant of multiple rheumatological disorders in addition to fibromyalgia syndrome such as generalized osteoarthritis, pseudogout, gout, rheumatoid arthritis, systemic lupus erythematosus, and polymyalgia rheumatica, as summarized in Table 123-2. A careful history and thorough physical examination for synovial and extrasynovial findings can help differentiate these conditions from fibromyalgia. Table 123-2 lists a number of key features on history and physical examination that may aid in the diagnosis. Other diagnostic considerations include hypothyroidism, vitamin D deficiency, demyelinating polyneuropathies, and paraneoplastic syndromes. A targeted laboratory panel may be helpful in teasing out these differential diagnostic considerations.
HISTORY | PHYSICAL EXAMINATION | ||||
|---|---|---|---|---|---|
DISORDER | AM Stiffness | Location of Pain | Synovitis | Extrasynovial Disease | OTHER DIAGNOSTIC FEATURES/COMMENTS |
Osteoarthritis | Generally short-lived, e.g., < 30 min | Weight-bearing appendicular joints, cervical and lumbar spine, DIPs, PIPs and first CMC. MCP and wrist involvement go against OA | Absent or mild | None related to arthritis itself. | Since OA is ubiquitous in older adults, x-rays should be used to rule out other disorders, not to diagnose OA. |
Pseudogout | Pseudorheumatoid pattern may be associated with prolonged AM stiffness | Knee and wrist are most common locations; disease is often symmetrical | Acute flares are intensely inflammatory | Chondrocalcinosis on x-rays; eye deposits, bursitis, tendonitis, carpal and cubital tunnel syndromes may occur. Tophaceous soft tissue deposits uncommon. | Chondrocalcinosis may be asymptomatic. Identification of intracellular CPPD crystals offers a definitive diagnosis in acute flares. Acute and chronic forms occur. |
Gout | Pseudo-rheumatoid pattern may be associated with prolonged AM stiffness | Joints of the lower extremities are most often involved, especially first MTP; disease is typically asymmetrical | Acute flares are intensely inflammatory | Tophi may deposit in soft tissues. | Hyperuricemia may be asymptomatic. Serum uric acid cannot diagnose gout. Identification of intracellular monosodium urate monohydrate crystals offers a definitive diagnosis in acute flares. |
Rheumatoid arthritis | Prolonged, e.g., > 30 min. Duration of stiffness is used as one parameter of disease activity | Any synovial joint. The lumbar spine is typically spared. | Present | Not uncommon; rheumatoid nodules can develop in soft tissues. Many other possible manifestations including anemia, vasculitis (skin lesions, peripheral neuropathy, pericarditis, visceral arteritis, palpable purpura), pulmonary disease, etc. | Patients may be seronegative. If disease is suspected, patient should promptly be referred to a rheumatologist to retard disease progression. |
Systemic lupus erythematosus | Not a prominent feature | Depends upon tissues involved—may or may not be limited to joints. Comorbid fibromyalgia is not uncommon. | Generally absent; arthralgias are more common than arthritis | Common—e.g., anemia, skin rash, pleuritis, peritonitis, pericarditis, nephritis, meningitis, etc. | Anyone with suspected SLE should promptly be referred to a rheumatologist. |
Fibromyalgia syndrome | Generally short-lived, e.g., < 30 min | Typically diffuse. Worst symptoms often involve the axial skeleton. | Absent. Joints themselves are not involved, although patients experience pain in joints and soft tissues | Many other disorders may coexist (see Table 123-4). | Fibromyalgia syndrome is not a diagnosis of exclusion, but one based upon careful history and physical examination (see text). |
Polymyalgia rheumatica | Maybe prolonged, lasting several hours | Typically proximal— e.g., shoulder girdle, hip girdle, neck. If headaches, jaw claudication, and/or prominent systemic symptoms (e.g., fever), consider temporal arteritis. | May occur, especially in small joints of hands | Occurs if comorbid temporal arteritis and relates to involvement of arteries (e.g., Raynaud’s phenomenon, bruits, claudication). | Because the erythrocyte sedimentation is very nonspecific, this test should be used to assist with confirmation of a suspected diagnosis. Note that cases of PMR and TA with a normal ESR have been reported. |
Vitamin D deficiency | Absent | Typically described as diffuse, deep pain. Bony pain is often present. | Absent | Fatigue is a common feature with proximal muscular weakness (pelvic-girdle myopathy). Tenderness with palpation of bony structures. | Fatigue and difficulty climbing stairs are common complaints. Gait imbalance and falls may be seen. In severe cases, the profound weakness results in need for a wheelchair. Radiologic findings include fractures and Looser-Milkman pseudofractures (osteomalacia giving a striped appearance to bones). Direct measurement of serum 25(OH)D is the best marker for vitamin D deficiency. |
Hypothyroidism | Absent | Diffuse myalgias and arthralgias. | Joint swelling may be present with noninflammatory joint effusions. Hand, knee, and wrist involvement. Avascular necrosis, gout and or pseudogout may co-exist | Myalgias, generalized weakness, and carpal tunnel may exist. | Fatigue, mental slowing, and depression often seen. Associated symptoms include hair loss, edema, cold intolerance, dry skin, constipation and weight gain. |
The characteristic physical examination finding in patients with fibromyalgia is the presence of tender points at the specific locations outlined in Table 123-3. A tender point is defined as a spot on the body that is painful with 4 kg of pressure (the amount of pressure required to blanch the examiner’s thumbnail when palpating the palm of her own hand). Palpation of the tender points with 4 kg of pressure is needed, as below this level, most subjects will not report pain. Those who report pain when tested with 4 kg of pressure demonstrate a lower than normal pain threshold. Patients reporting pain in fewer than the 11 of 18 tender points included in the American College of Rheumatology classification criteria may still be diagnosed with fibromyalgia if they have otherwise supportive clinical features (e.g., sleep disturbance, fatigue, morning stiffness). Tender points exist in a number of conditions other than fibromyalgia, including cervical and lumbosacral facet arthrosis syndrome, sacroiliac joint syndrome, and chronic whiplash and therefore a careful history and examination are key diagnostic elements.
FINDING | OPERATIONAL DEFINITION | EXAMINATION TECHNIQUE |
|---|---|---|
Fibromyalgia tender points | Presence of pain when approximately 4 kg of force (i.e., enough force to blanch examiner’s thumbnail bed) is applied to defined tender points | Have patient sit comfortably on examination table, arms resting in lap. Tell patient that you are going to apply pressure at several points on the body, and that you want to know if pressure on any point causes pain. Examine the following points bilaterally, using enough pressure to blanch thumb nail:
|
Functional leg length discrepancy | Pelvic asymmetry | Have patient stand with both feet on floor, shoes removed. Ask him to stand with feet together, and as erect as possible. Kneel behind patient. With palms parallel to floor, and fingers extended, place lateral surface of index finger of both hands atop pelvic brim bilaterally. Level of eyes doing the examination should be level with hands. Determine if right and left hands are at different heights. |
Scoliosis (lateral/rotational) | Lateral/rotational curvature of thoracolumbar spine | Have patient stand on floor with shoes removed. Stand behind patient. Run index finger along spinous processes (do not lift hand between vertebrae) a series of 3 times. If you do not detect scoliosis, then: Ask patient to bend forward. Determine if there is asymmetry in height of paraspinal musculature. |
Sacroiliac joint pain | Pain with direct palpation of sacroiliac joint or with Patrick’s test | Direct palpation: Have patient stand on floor with shoes removed. Stand behind patient. Exert firm pressure over sacroiliac joint, first on one side, then the other. Palpate right joint with right thumb, standing to left side of patient; palpate left joint with left thumb, standing to right of patient. Patrick’s (Fabere) test—Have the patient lie supine on the examining table and place the foot of involved side on opposite knee Then slowly lower the test leg in abduction toward the examining table If patient reports pain in back (not groin, buttocks or leg), then test is positive |
Myofascial pain, piriformis | Presence of pain on deep palpation of piriformis | Have patient lay supine on examination table Have patient flex right hip and knee, keeping sole of foot on table Cross bent leg over opposite leg; again place sole on table and exert mild medially directed pressure on lateral aspect of knee to put piriformis in stretch Exert firm pressure (4 kg) over middle extent of piriformis Repeat examination on opposite side |
Myofascial pain, TFL ± iliotibial (IT) band pain | Presence of pain on deep palpation of tensor fascia lata and/or IT band | Have patient lying supine on examination table. Using thumbs of both hands, exert firm pressure (4 kg) over full extent of TFL and IT band. Repeat examination on opposite side. |
Kyphosis | Deformity of thoracic spine creating forward flexed posture |