Overview
Pregnancy is a complex physiological state consisting of a symbiotic relationship between two genetically distinct, but related, individuals: the mother and the fetus. The success of pregnancy requires dramatic alterations of maternal physiology to accept, protect, house, and nurture the fetal allograft. A successful pregnancy involves implantation of the developing embryo into the endometrium; its avoidance of immunological rejection by the maternal immune system; adaptation of the maternal uterus to sustain a pregnancy; and specific changes in maternal physiology to meet the nutritional, metabolic, and physical needs of the growing conceptus—along with the proper timing of parturition, so that birth occurs when the fetus is mature enough to survive outside the uterus and the mother is able to nurture her newborn child. Hormonal interactions between the fetal and maternal compartments control these processes. A unique and extremely important endocrine organ of pregnancy is the placenta. The hemochorial anatomy of the human placenta allows direct access of the fetal syncytiotrophoblast to the maternal circulation for hormonal secretion. Placental hormones flood the maternal systems and act on maternal target cells to adjust maternal physiology in favor of maintaining pregnancy and meeting fetal metabolic needs. Through this endocrine relationship, the fetus and mother maintain homeostasis and allows the fetus necessary time for growth and functional development. Defects in placentation, placental function, and aberrations in placental hormone production cause maternal and newborn pathophysiology, such as life-threatening preeclampsia and fetal growth restriction. The end of pregnancy usually occurs through the process of parturition and, in most cases, at a time, referred to as term , when the fetus is sufficiently mature to survive as a neonate. Term for human gestation is between the 37th and 42nd completed week of gestation (measured from the last menstrual period). Preterm birth, defined as less than 37 completed weeks of gestation, is a major worldwide socioeconomic problem that accounts for the majority of neonatal morbidity and mortality. The hormonal control of parturition—such that it occurs at term—is therefore a major determinant on neonatal wellness and the success of pregnancy. This chapter addresses current understanding of the endocrinology of pregnancy and parturition from the perspective of fetal/placental wellness and the process and timing of parturition, including recent advances in unraveling the genetics of human gestation length and birth timing. The discussion will be prefaced by a contextual overview of the evolutionary biology of human pregnancy and birth timing.
Evolution of Human Pregnancy and Birth
Pregnancy is both a personal event between mother and baby and a sociological and public health concern, as is evidenced by high infant and maternal mortality rates, even in developed countries like the United States. Although scientific advances have allowed us to learn about potential causes of preterm birth and pregnancy complications, it is critical to look back, through an evolutionary lens, to more fully understand the process of pregnancy and birth.
Evolution optimizes reproductive fitness across generations—maximizing transmission of genes to the next generation. Pregnancy and parturition are key events in the reproduction of viviparous species and, as such, would have been subjected to strong selective pressure through evolution. Advances in genetic technologies allow in-depth examination of the influence of evolutionary dynamics on pregnancy—including the impact of genetics and hormones on conception, pregnancy maintenance, and triggers for parturition. Ultimately, genetic makeup reflects natural selection and, as such, evolutionary adaptations may contribute to extant pregnancy complications, because the current genetic makeup of the mother, or even the baby, may not be ideal for current environmental conditions. This mismatch may be the reason for diseases of pregnancy, such as preeclampsia and gestational diabetes—and, in fact, likely influences health throughout the life span.
“Nothing in biology makes sense except in light of evolution,” said geneticist and evolutionary biologist Theodosius Dobzhansky. This concept is certainly reflected in the comparative biology of pregnancy and parturition among viviparous species. The common theme among viviparous species is that the fetal development requires a minimum gestation time and a supply of nutrients for the fetus to achieve functional maturity needed to survive as a neonate. Fetal organ systems (especially the pulmonary, renal, and gastrointestinal) and neuroendocrine axes (especially the hypothalamic-pituitary-adrenal [HPA] and thyroid systems) must be sufficiently developed at birth for the newborn to achieve and maintain homeostasis. Similarly, maternal physiology, which has been modified to provision pregnancy, must be prepared to nurture and protect the newborn. Certain processes are common (e.g., the requirement for progesterone to establish and maintain pregnancy; the promotion of fetal organ maturation by glucocorticoids) to all species, but subtle differences exist that relate to specific selective pressures on pregnancy-related traits that improved reproductive efficiency against the backdrop of general habitus, environmental niche, and overall reproductive strategy.
Viewing conception through an evolutionary lens begs the question: Why doesn’t the mother’s immune system attack the fetus? The “inflammation paradox” hypothesis posits that inflammation may be a necessary process that evolved to aid implantation, rather than attack the fetus. This is just one part of the equilibrium that must be established in a healthy pregnancy between the needs of the mother and the needs of the fetus. This concept has been described as maternal-fetal “cross-talk,” “tug-of-war,” or a “modulation” of gene regulation or cell networks. This often-conflicting cadence between mother and fetus continues throughout pregnancy, highlighting nutritional needs and hormonal fluctuations that must remain balanced for a successful pregnancy to carry to term. Recent studies of individual cells at the maternal-fetal interface have identified regulatory interactions at the cell level between the mother and fetus that prevent immune cell attack of the conceptus. Through an evolutionary framework, preeclampsia, for example, could be the result of shallow trophoblast invasion and inadequate spiral artery remodeling—forcing the fetus to increase maternal blood flow to the placenta by increasing maternal peripheral vascular resistance and providing greater blood flow to the fetal interface.
Another “obstetric dilemma” is conferred by the combined traits of encephalization: the increase in brain mass relative to body mass that is unique to hominid lineages, and obligate bipedalism. Bipedalism evolved between 3 to 5 million years ago, leading to an increase in hominid brain size, estimated at about 1 million years ago. The size of the human brain has remained relatively constant for at least the last 100,000 years. Obligate bipedalism required changes in the pelvic anatomy that decreased the size of the obstetric outlet. This would have limited the extent of intrauterine encephalization and required that parturition occur before the fetal head becomes larger than the pelvic outlet. This trait appears to be unique among human lineages. No other primates face this level of cephalopelvic disproportion, as they have pelvic openings that are larger than the fetal head diameter. Women, however, must pass a large-headed fetus through a relatively small pelvic opening. This means that the fetus cannot grow too large, or it risks death for the baby and the mother. Evolutionary selection of ancestral lineages must have been at work to develop a gestation length optimal for fetal development, especially lung maturity, before parturition. In addition, recent research has found regional and ancestral variability in the shape of birth canal. Strict evolutionary theory might surmise that the birth canal should be similarly shaped. Instead, this new work shows that other evolutionary forces, such as genetic drift, migration patterns, or even climatic adaptation also may have been at work. Study authors Lia Betti and Andrea Manica state that thoroughly understanding this has implications for obstetric practice in multiethnic societies.
Rapid fetal brain growth requires substantial energy, and therefore traits that increase energy transfer from the mother to the fetus would have been favored. This may explain the production of placental lactogen and placenta growth hormone (GH) that promote maternal insulin resistance, providing more glucose for fetal consumption. However, traits that favored fetal brain growth would have conflicted with the problem of birthing a large-headed fetus through a relatively small outlet. This problem may have been solved by altering the parturition timing mechanism to shorten gestation thereby avoiding the obstetric dilemma . Allometric analyses of neonatal brain size to body ratios across primate species, and the fact that human neonates are altricial (referred to as secondary altriciality ), whereas other extant primates are precocial, support the hypothesis that gestation was shortened in modern-day hominids. This is a reasonable hypothesis that explains much of the unique characteristics of human pregnancy and parturition. As natural selection generally operates at the population level and over many generations, traits that increase reproductive efficiency of the population (such as encephalization; fetal neuroendocrine plasticity) and are beneficial over the long term, may impart costs to an individual, such as increased risk for preterm birth or cardiovascular disease over the short term.
Consideration of these traits through an evolutionary lens expands our perspective of pregnancy complications, such as preeclampsia, fetal growth restriction, gestational diabetes, and preterm birth, and normal processes, such as the plasticity of fetal neuroendocrine development in response to environmental cues.
Establishment of Pregnancy
Under normal circumstances, implantation of a viable embryo into a receptive uterine endometrium establishes pregnancy. During the menstrual cycle, the ovarian steroid hormones, estradiol and progesterone, induce structural and functional changes in the endometrium essential for the establishment of pregnancy. During the luteal phase, the endometrium converts to a secretory phenotype in response primarily to progesterone produced by the corpus luteum (CL). The secretory endometrium is spongy and highly vascularized and has a glandular epithelium that secretes factors into the uterus that favors embryo survival. At the same time, epithelial cells in the endometrium produce chemokines, growth factors, and cell adhesion molecules that attract the embryo to specific docking sites for implantation and, in addition, increased vascularization of the endometrial stroma provides an optimal substrate for placentation.
After fertilization, the zygote undergoes an intrinsic program of cell division and differentiation that is not dependent on the hormonal milieu of the fallopian tube or the uterus. At around the fourth day after fertilization, the embryo is a solid cluster of cells encapsulated by the remnant of the oocyte zona pellucida. In the next 24 to 48 hours, as the embryo moves through the fallopian tube toward the uterus, it develops a fluid-filled cavity, the blastocele, and is referred to as a blastocyst . The outer layer of blastocyst cells, known as the trophectoderm , will give rise to the placenta and chorionic membrane. An inner cell mass of the blastocyst will produce the fetus, amnion, and mesenchymal and vascular components of the placenta. The human blastocyst enters the uterus at around the fifth day after fertilization and floats freely in the uterine cavity for 2 to 3 days. By this stage, the zona pellucida degenerates, leaving the nascent blastocyst embryo in an optimal condition to implant into the endometrium.
Embryo implantation into the endometrium has temporal and spatial limits within the receptive uterus. Physical interaction of the blastocyst with the endometrial epithelium occurs at dome-like structures known as pinopodes that express chemokines and cell adhesion molecules that attract the embryo and appear to be the preferred site for embryo adherence and subsequent implantation. Embryo adherence and implantation is most successful between days 21 and 24 of the menstrual cycle (~ 85% success rate), whereas implantation before or after this optimal window has a low (~ 11%) success rate. After day 24 to 25, the endometrial stroma undergoes morphologic and functional changes collectively referred to as decidualization , which is dependent on progesterone and occurs independently of conception and implantation. The decidualized endometrium, composed of large polyhedral cells, containing high levels of glycogen and lipids that secrete a tough pericellular capsule, is hostile to implantation. Therefore successful implantation must occur before the establishment of the decidual barrier. In an infertile cycle, menstruation occurs to eliminate the nonreceptive decidualized endometrium. In the subsequent cycle, the endometrium then renews to a receptive state.
For implantation, trophoblast cells proliferate and secrete proteases that degrade the extracellular matrix between endometrial cells forming a path for the blastocyst to enter the uterine stroma. The invading trophoblast cells are referred to as cytotrophoblasts ( CTBs ) and form columns penetrating to the basal membrane beneath the decidual cells and into the myometrium. Eventually, the entire embryo embeds in the uterine stroma, anchored by CTB columns. Some extravillous CTBs invade into maternal spiral arterioles, displacing endothelial cells and smooth muscle tissue, and dilating the vessels to create a low-resistance arteriolar system. This system directs maternal blood into spaces, known as lacunae , between the invading columns of CTB cells. During this time, some CTBs fuse their plasma membranes to become a syncytiotrophoblast . This becomes the outer layer of the functional placenta. Eventually, the syncytiotrophoblast forms schorionic villi that are bathed in maternal blood within the lacunae.
The embryo is semiallogeneic with respect to the mother, and therefore its invasion into the endometrium represents a major breach of the maternal immune system. For successful pregnancy, the placental syncytiotrophoblast and CTBs at the maternal-fetal interface must avoid attack and destruction by activated maternal immune cells. To this end, the trophoblast cells produce an immunogenic camouflage in the form of human leukocyte antigen (HLA)-G that has reduced polymorphism; therefore maternal immune cells do not recognize it as foreign. CTBs also express multiple factors: Fas ligand that induces apoptosis in immune cells that carry Fas receptor, early pregnancy factor, and the progesterone-induced blocking factor that blocks lytic natural killer (NK) cell activity and several antiinflammatory cytokines (such as transforming growth factor [TGF]-ß, interleukin [IL]-10, IL-4).
For the establishment of pregnancy, menstruation that normally occurs during a nonfertile cycle must be prevented. This happens when the trophoblastic cells of the early embryo secrete chorionic gonadotropin (CG) that prevents CL regression and maintains its secretion of progesterone. In nonfertile cycles, the CL usually regresses at about the second week after ovulation, and the subsequent decline in progesterone leads to menstruation. Thus one of the first endocrine interactions between the conceptus and the mother involves early embryo signaling to promote intrauterine conditions that will allow implantation and the establishment of pregnancy via maintenance of CL progesterone secretion.
Endocrine Placenta: Structure and Function
The placenta fulfills a variety of essential functions during prenatal life. Its global role is to maintain a protected environment that facilitates optimal growth and development of the embryo and fetus. The human, hemochorial placenta includes a chorionic plate and chorionic villi. The chorionic villi consist of stem villi (types 1 to 3) either anchored to the decidua basalis or floating free. Type 3 villi branch out into intermediate and finally terminal villi. The surface of the terminal villi is covered in CTB progenitor cells, derived from the trophectoderm of the early blastocyst, and anchored to a basal lamina. The low oxygen environment of the early placenta protects organogenesis and favors CTB proliferation. However, as development progresses, differentiation of these trophoblast progenitor cells produces different cell phenotypes.
CTBs differentiate into an invasive phenotype, and extravillous trophoblasts (EVTs) invade the uterine wall anchoring some chorionic villi to the decidua basalis entering the maternal blood vessels. These invading CTBs plug maternal arterioles maintaining the state of physiological hypoxia. EVTs continue to move further up maternal spiral arterioles, replacing maternal endothelial cells, remodeling, and increasing the diameter of the arterioles to accommodate the massive increase in blood supply required for fetal growth. Furthermore, several maternal cell types maintain the balance between inflammation and tolerance in the decidua. In particular, innate lymphoid cells, such as NK cells, interact with trophoblasts, stromal cells, and neutrophils to play a key role in the induction and maintenance of pregnancy. Decidual NKs represent 50% to 70% of infiltrating lymphocytes during the first trimester, but numbers diminish throughout gestation. Decidual NK cells are involved in early remodeling of the maternal spiral arteries before trophoblast invasion, as well as secreting chemoattractant factors critical for EVT migration and invasion (such as IL-8, CXCL10, and IL-6). An association exists between impaired trophoblast chemotaxis and improper spiral artery remodeling and abnormal placentation. Abnormal decidual NK (dNK) function also may lead to a loss of control of trophoblast invasion.
During this time, some CTBs fuse their plasma membranes to become a single multinucleated cell known as the syncytiotrophoblast , which becomes the outer layer of the functional placenta. By 10 to 12 weeks of gestation, the CTB plugs are broken down and maternal blood reaches the intervillous spaces (lacunae), resulting in a change in oxygen tension in the placenta and the syncytiotrophoblast comes into direct contact with maternal blood in the lacunae. Remodeling of the placental villi and their associated underlying vasculature, which connects to the umbilical cord, occurs throughout gestation and can be responsive to maternal signals/environmental exposures impacting placental function, fetal growth, and development, and potentially, disease development later in childhood and adulthood (a process known as fetal programming ).
The placental syncytiotrophoblast facilitates oxygen and nutrient transfer from the maternal circulation to the fetus and disposes of fetal waste products. It synthesizes and secretes hormones, growth factors, cytokines, and other bioactive molecules mainly into the maternal compartment. It also metabolizes maternal hormones (such as glucocorticoid and insulin) to prevent fetal exposure, and thus, separates components of the maternal and fetal endocrine systems. Importantly, the hemochorial arrangement allows hormones produced by the syncytiotrophoblast to directly access the maternal circulation. In contrast, the syncytiotrophoblast prevents most maternal hormones from entering the fetal compartment. Most hormones the placenta produces are identical, or close structural and functional homologues, of existing maternal hormones. As such, they interact with cognate receptors on maternal cells. Consequently, placental hormones that are secreted in relatively large amounts to achieve high levels (compared with levels in the nonpregnant women) in the maternal circulation override maternal counterparts and have profound effects on maternal physiology. Placental hormones include members of the prolactin and GH family, steroid hormones, and neuroactive hormones.
Prolactin-Growth Hormone Family
The prolactin-growth hormone (PRL-GH) family is one of the major groups of hormones the placenta secretes during gestation. Members of this family consist of prolactin, placental lactogens, and GH. Their roles include mediating maternal metabolic adaptations to pregnancy. The placenta’s production of the PRL-GH family of hormones appears to be important in regulating both insulin production and the sensitivity of the mother in response to pregnancy. The PRL-GH family also is implicated in the regulation of appetite and body weight.
Steroid Hormones
The placenta is the primary source of steroid hormones during gestation. Placental steroid hormones include estrogens and progesterone. Both estrogen and progesterone play roles in regulating insulin and glucose homeostasis, lipid handling, and appetite regulation, which may be important in promoting metabolic changes and mobilizing nutritional stores in the mother during pregnancy. Steroid hormones are implicated in pregnancy complications, such as gestational diabetes and preeclampsia. High progesterone and estrogen concentrations have been reported for women with gestational diabetes, whereas levels are reduced in preeclamptic pregnancies compared with healthy pregnancies.
Neuroactive Hormones
Major targets of placental hormones include the maternal brain, hypothalamus, and pituitary glands. These neuroendocrine effects enable the mother to respond to her environment and adapt to avoid adverse effects of stress and maintain homeostasis. Neuroactive hormones also prepare and enable the future mother to adequately care for her offspring. Melatonin and its precursor, serotonin, are tryptophan-derived hormones with well-known neuroendocrine impacts. During gestation, circulating levels of melatonin and serotonin increase. The placenta also secretes neuroactive hormones, such as thyrotropin-releasing hormone (TRH) and kisspeptin, which may function in adapting maternal physiology to support pregnancy. The placenta secretes oxytocin and expresses its receptor. Both increase gradually in the late stage of pregnancy in the normal placenta.
Corticotropin-Releasing Hormone
The human placenta expresses the gene encoding corticotropin-releasing hormone (CRH) starting around the sixth to eighth week of gestation. Placental CRH can be detected in the maternal blood by the 15th week of gestation with levels increasing exponentially up to the time of delivery. For most of pregnancy, CRH circulates in association with a binding protein, which sequesters CRH and prevents its biological activity. At the end of pregnancy (4–5 weeks before parturition), levels of the CRH binding protein in the maternal blood fall. This is associated with an exponential increase in placental CRH production, resulting in increased CRH biological activity. Placental CRH production (based on in vitro studies of placental explants) is increased by prostaglandins (PGs) E 2 and F 2α , norepinephrine, acetylcholine, vasopressin, angiotensin-II, oxytocin, IL-I, glucocorticoids, and neuropeptide-Y, and decreased by progesterone and nitric oxide donors.
Growth Factors
The human placenta secretes multiple growth factors and cytokines. Growth of the placenta involves trophoblast proliferation, migration, differentiation, and fusion, and as such, growth factors are likely to be involved in these processes. Development of the placenta also involves extensive angiogenesis and vascularization at the implantation site, and modulation of the maternal immune system to prevent rejection of the allogeneic fetal tissue. These processes likely involve a complex autocrine/paracrine communication that involves a plethora of growth factors, angiogenic factors, and cytokines.
Insulin-Like Growth Factors
The human placenta produces insulin-like growth factor (IGF)-1 and IGF-2 from as early as the eighth week of gestation. IGF-1 is present in syncytiotrophoblast and CTBs at all stages in gestation. IGF-2, plays a role in modulating maternal sensitivity to glucose, insulin, and glucose levels. Abnormal IGF-1 expression, levels, and signaling are highly associated with intrauterine growth retardation in humans.
Vascular Endothelial Growth Factor Family
CTBs, the syncytiotrophoblast, and villous stromal cells in the placenta express members of the vascular endothelial growth factor (VEGF) family of peptides. Placental growth factor (PGF) is a member of this family, which shares about 50% homology with VEGF-A. It is expressed by villous and extravillous trophoblast cells, and in contrast to VEGF expression levels, are positively correlated with advancing gestation. Imbalance in circulating maternal levels of these peptides and their soluble receptor, soluble FMS-like tyrosine kinase (sFLT), is strongly, but not exclusively, associated with the etiology of preeclampsia. However, the contribution to these levels from maternal endothelium is unclear. Moreover, CTB and villous stromal expression of these peptides regulates villous vascular formation and remodeling throughout pregnancy, and if disrupted, impacts transfer of nutrients and oxygen into the fetal circulation.
Fibroblast Growth Factor Family
Villous trophoblasts and villous mesenchyme in the human placenta express members of the fibroblast growth factor (FGF) family, and placental mononuclear phagocytes (also known as Hofbauer cells ) express FGF receptors. FGF induces Hofbauer cells to produce multiple growth factors and cytokine involved in tissue repair.
Adiopokines
The human placenta expresses adipokines, leptin, adiponectin, resistin, ghrelin, and visfatin, and secretes them into the maternal circulation. They are thought to regulate maternal metabolic adaptation to pregnancy, especially increased insulin resistance. In placental cells, leptin and adiponectin modulate trophoblast invasiveness and the nutrient supply. Leptin is inversely correlated with birth weight in small-for-gestational-age newborns. Positive correlation of adiponectin with leptin and ghrelin expression suggests an interaction between these hormones in the placenta.
Transforming Growth Factor-ß Family
TGF-ß influences both the proliferation and differentiation of trophoblast cells acting through receptors that include endoglin (ENG), a coreceptor for TGF-ß1 and TGF-ß3, which the syncytiotrophoblast and endothelial cells express. Trophoblast cells produce a soluble truncated ENG (sENG) consisting of the extracellular domain. sENG expression is upregulated in preeclampsia and may act as a decoy to sequester and block TGF-ß1 binding to its endothelial cell receptor, preventing vasodilation. sENG appears to augment the endothelial dysfunction caused by elevated sFLT1.
Activins and inhibins are disulfide-linked homo- and heterodimeric members of the TGF-ß family that derive their names from their ability to activate or inhibit, respectively, pituitary follicle-stimulating hormone (FSH) secretion. The syncytiotrophoblast expresses each of the activin/inhibin subunits and follistatin, and the levels of expression do not change with advancing gestation. These factors are secreted into the maternal and fetal circulations and amniotic fluid and their production varies with stage of gestation. Activins and inhibins may affect placental CG production. In cultured trophoblast cells, inhibin suppresses gonadotropin- releasing hormone (GnRH)–induced CG expression, whereas activin augments the GnRH-induced release of CG. Thus at least in vitro, activin and inhibin via paracrine effects on placental GnRH production may contribute to the regulation of CG secretion in a manner similar to their effect on hypothalamic-pituitary gonadotropin secretion. Interestingly, levels of inhibin-A and activin-A in the maternal circulation have been reported to have predictive value for pathologies, such as placental tumors, hypertensive disorders of pregnancy, intrauterine growth restriction, fetal hypoxia, Down syndrome, fetal demise, preterm delivery, and intrauterine growth restriction.
Epidermal Growth Factor Family
CTBs and the syncytiotrophoblast in the human placenta express epidermal growth factors (EGFs) early in pregnancy, and the level of expression decreases with advancing gestation. EGF is thought to promote trophoblast invasion during implantation and deficiency in EGF expression and/or signaling is associated with preeclampsia and fetal growth restriction.
Extracellular Vesicles
The placenta produces extracellular vesicles (EVs) in large quantities in both healthy and pathological pregnancies. EVs now are being recognized as important carriers for proteins, lipids, and nucleic acids, which may play a crucial role in fetomaternal communication and maternal adaptation to pregnancy. Micro- and nanovesicles from both first trimester and term human placentae carried Flt-1, and levels significantly increased in EVs from severe, but not mild, preeclampsia compared with normotensive placentae. Placental exosomes can be detected in the maternal circulation from the sixth week of gestation, and their levels increase gradually with advancing gestation in proportion to the increased size of the placenta. Microribonucleic acid (microRNAs) in placental exosomes affect the function of local immune cells to boost resistance to viral infection. Levels of exosomes (placental and nonplacental) in the maternal circulation, and the composition of their cargo, have been associated with pregnancy complications, such as preeclampsia, preterm birth, intrauterine growth restriction, and gestational diabetes.
Cell-Free Fetal Nucleic Acids
Cell-free deoxyribonucleic acid (cfDNA) originates in trophoblast cells and can be assayed in the maternal blood as biomarkers of placental health and function. From around the seventh week of gestation, cfDNA is detectable in the maternal circulation, and the amount increases with advancing gestation, such that late in pregnancy, it represents around 4% of the cfDNA in maternal blood. After delivery of the placenta, the amount of cfDNA in maternal blood rapidly decreases. The physiologic role of cfDNA—and why it is shed from trophoblast cells into the maternal compartment—is not known. The amount of cfDNA in maternal blood has been used as a biomarker of placental health, with increases in cfDNA noted in nonviable pregnancies, preeclampsia, and intrauterine growth restriction-associated with placental insufficiency.
Because it can be isolated and sequenced, cfDNA is used for noninvasive prenatal diagnosis and testing for conditions, such as X-linked genetic disorders and aneuploidies, as well as fetal sex determination.
Cell-free messenger RNA (mRNA) derived from the placenta has been detected in maternal blood by eight weeks of gestation. The cell-free mRNA has been evaluated quantitatively for specific transcripts, particularly those encoding proteins expressed uniquely by the placenta (such as CG β-subunit, placental lactogen, placenta enriched -1 14). It has been found that some of these transcript levels in maternal blood are elevated in preeclampsia and intrauterine growth restriction.
MicroRNAs, short noncoding single-strand RNA molecules, are known to be epigenetic factors regulating gene expression by targeting specific sequences in mRNAs and destabilizing the transcripts and/or inhibiting or reducing translation. The microRNAs released from the placenta may be incorporated into microvesicles, including exosomes, in apoptotic bodies, or protein-bound. The placenta-derived microRNAs are thought to influence maternal gene expression, particularly in vascular endothelial cells. The placental-specific microRNAs include those encoded in clusters on chromosomes 14 and 19, with the best studied being the microRNAs from the chromosome 19 cluster (C19MC). Alterations in levels of specific microRNAs in maternal blood have been reported in nonviable pregnancies and preeclampsia.
Fetal Neuroendocrine Development
The neuroendocrine system, including the hypothalamus, anterior pituitary, and the major endocrine glands, is essential for physiologic homeostasis, growth, response to stress, and reproduction. Appropriate development of the neuroendocrine systems during fetal life is therefore critical for postnatal health and wellness.
The neuroendocrine system functionally develops by midgestation. The hypothalamus originates on the inner surface of the diencephalic neural canal at around the sixth week of gestation. By the tenth week, the median eminence can be distinguished and specific cells in the hypothalamus begin producing GnRH, TRH, CRH, GH-releasing hormone (GHRH), and somatostatin (SS). The primordium of the anterior pituitary appears at around the fourth week of gestation as an evagination of Rathke’s pouch, in front of the buccopharyngeal membrane at the roof of the developing buccal cavity. By the seventh week, the floor of the sella turcica is in place and separates the anterior pituitary from its epithelial origins. Beginning during week 8, capillaries interdigitate among the mesenchymal tissue of Rathke’s pouch and the median eminence of the hypothalamus, and by the 12th to 15th week of gestation, these vessels form the hypothalamic-hypophyseal vascular system. The mature anterior pituitary gland includes lactotropes that produce prolactin (PRL), somatotropes that produce GH, corticotropes that produce proopiomelanocortin (POMC) and that give rise to adrenocorticotropic hormone (ACTH), ß-lipotropin and ß-endorphin, thyrotropes that produce thyroid-stimulating hormone (TSH), and gonadotropes that produce luteinizing hormone (LH) and FSH. Thus the fetal hypothalamic-pituitary axis is anatomically and functionally developed by midgestation and, for the remainder of pregnancy, the fetal anterior pituitary, under the control of the hypothalamus, produces tropic hormones and affects the fetal thyroid glands, adrenal glands, and gonads. Although, each endocrine axis exhibits varying levels of activity, the fetal HPA axis is remarkably active in preparation for its essential role postnatally.
Integrative Steroidogenesis During Pregnancy: Fetal Adrenal-Placental Crosstalk
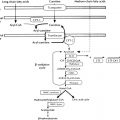
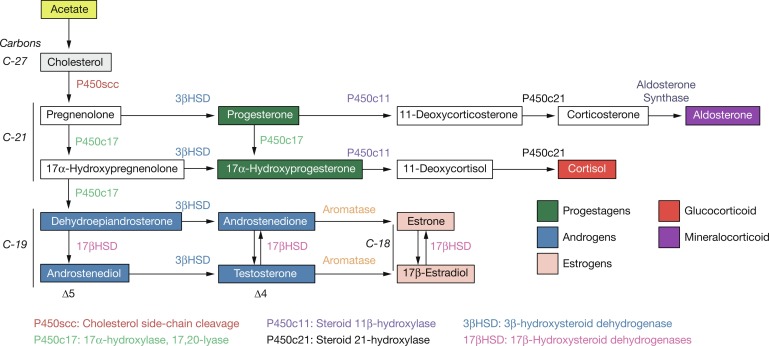
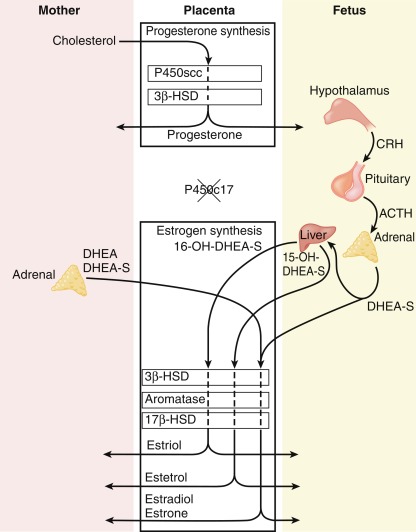
The human placenta produces large amounts of progesterone and estrogens, especially after the first trimester. At 6 to 10 weeks of gestation, placental trophoblast cells and the syncytiotrophoblast gain the capacity to convert maternal cholesterol to progesterone. Placental cells also have high aromatase and 17ß hydroxysteroid dehydrogenase (17ßHSD) activity and convert C19 androgens to C18 estrogens ( Fig. 5.1 ). However, the placenta cannot produce estrogens de novo from cholesterol, or from pregnenolone or progesterone because the cells lack the 17α-hydroxylase/17,20-lyase enzyme. Instead, the fetal adrenal cortex supplies C19 androgens. Thus the human placenta produces progesterone from maternal cholesterol (referred to as the maternal-placental endocrine unit ) and estrogens from fetal adrenal C19 precursors (referred to as the fetoplacental endocrine unit ) ( Fig. 5.2 ). Deficiency in placental aromatase may not affect implantation, but women report virilization in the third trimester, resulting in facial hair and acne. Female newborns had varying degrees of pseudohermaphroditism with clitoromegaly and hypospadias—resulting from the inability of the placenta to convert dehydroepiandrosterone (DHEA) to estrogen. Male offspring developed tall stature because of failure of epiphyseal fusion, along with delayed bone age, causing osteopenia and undermineralization, along with several other issues.
The human adrenal glands derive from a thickening of the celomic epithelium between the urogenital ridges that occurs at around the fourth week of gestation. Approximately 1 week later, the primordial adrenal cortical cells migrate toward the mesonephros where they aggregate and proliferate to form the anlage of the adrenal cortex. For most of pregnancy, the fetal-adrenal cortex displays two morphologically distinct zones: the large inner fetal zone and the narrow outer definitive zone. A third zone, the transitional zone, distinguished by ultrastructural and functional characteristics, exists between the fetal and definitive zones and is apparent early in the third trimester ( Fig. 5.3 ). Thus late in pregnancy, the fetal-adrenal cortex resembles a rudimentary form of the adult-adrenal cortex. Soon after birth, the fetal zone involutes to become the zona reticularis, the transitional zone becomes the zona fasciculata, and the definitive zone becomes the zona glomerulosa. Between the innermost fetal zone cells—that eventually will aggregate to form the adrenal medulla late in the third trimester—are clusters of immature neuroblasts.
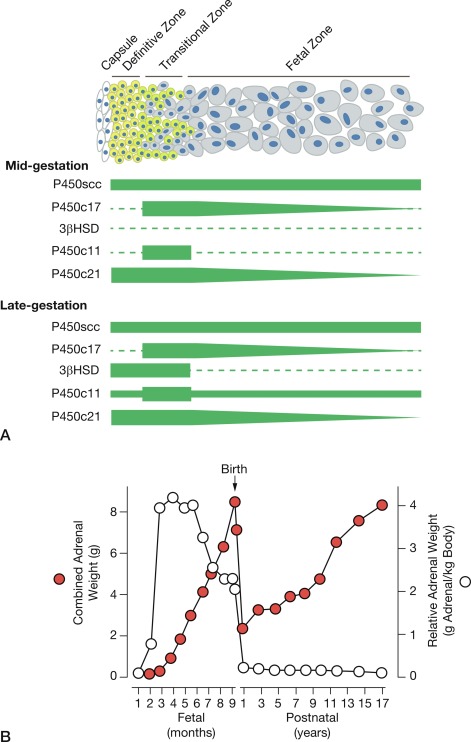
At around week 12 of gestation, steroidogenesis in response to ACTH initiates in the fetal zone cells. This increase coincides with the initiation of fetal zone enlargement and the fetal pituitary gland secreting ACTH. At midgestation, the fetal zone occupies 80% to 90% of the cortex and is the primary site of growth and steroidogenesis production. Remarkably, the adrenal glands are disproportionately enlarged because of fetal zone hypertrophy, and at 20 weeks, are larger than their adjacent kidney. Fetal zone cells are highly steroidogenic and in response to ACTH produce 100 to 200 mg/day of dehydroepiandrosterone sulfate (DHEA-S), a C19 steroid with weak androgenic activity, during the third trimester. The fetal adrenals produce DHEA-S that is used as substrate for placental estrogen synthesis. In the placenta, the steryl-sulfatase enzyme removes the sulfate and the 3ßHSD, 17ßHSD, and aromatase enzymes convert DHEA to estradiol and estrone. The fetal liver expresses the 16-hydroxylase enzyme and converts some DHEA-S to 16-hydroxy-DHEA-S (16OH-DHEA-S), which is then converted to estriol. Because 16-hydroxylase is not expressed postnatally, the amount of estriol in the maternal circulation indirectly reflects activity of the fetal HPA axis. For this reason, maternal estriol previously was used as an endocrine marker to evaluate fetal well-being. The fetal adrenal cortex continues to produce DHEA-S in response to ACTH for the remainder of pregnancy, increasing considerably during the second and third trimesters. By term, the human fetal adrenal produces around 200 mg DHEA-S per day. Thus the fetoplacental endocrine unit constitutes a cooperative interaction between the incomplete steroidogenic pathways in the fetal HPA axis and the placenta to produce a complete estrogen synthetic unit. Initial reports of steroid sulfatase deficiency suggested delayed onset of labor and lack of cervical dilation. However, subsequent research has shown normal gestation length with the possibility of spontaneous vaginal delivery.
The placenta’s production of CRH also influences the fetal HPA axis. A unique feature of primate pregnancy is that the placenta produces CRH in increasing amounts with advancing gestation. Placental CRH affects activity of the fetal HPA axis via a positive feedback endocrine loop, whereby the fetal adrenals produce cortisol to increase the placenta’s secretion of CRH. In turn, that stimulates the fetal anterior pituitary to further secrete ACTH to drive more cortisol synthesis. CRH also appears to increase ACTH responsiveness of cells in the transitional zone directly affecting adrenal cortical cells. As a result, fetal adrenal cortisol and placental estrogen synthesis may increase through a positive feedback loop involving placental CRH.
The fetal adrenal glands must be functionally mature to produce glucocorticoid (cortisol) and mineralocorticoid (aldosterone) immediately after birth so that the neonate can maintain homeostasis. Impairment of cortisol and aldosterone secretion postnatally can be fatal. An example of such a condition is congenital adrenal hyperplasia (CAH), a constellation of genetic defects in which the adrenal glands cannot produce cortisol and aldosterone. In this condition, the lack of cortisol negative feedback to the hypothalamus-pituitary leads to increased production of ACTH that induces overgrowth of the adrenal glands. The condition arises from mutations in any of the genes encoding key steroidogenic enzymes involved in cortisol and aldosterone synthesis. These include mutations in CYP21A2 (encodes 21-hydroxylase), HSD3B2 (encodes adrenal 3ßHSD), CYP11B1 (encodes 11ß-hydroxylase), POR (encodes P450 oxidoreductase), CYP17A1 (encodes 17α-hydroxylase), and STAR (encodes steroidogenic acute regulatory protein that mediates the transportation of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane, where it can be used for the synthesis of pregnenolone). CAH caused by 21-hydroxylase deficiency (referred to as classic CAH ) is the most common cause of adrenal steroid hormone insufficiency in pediatric patients. This defect impairs the synthesis of cortisol and aldosterone, whereas C19 androgen synthesis is not affected and dramatically increases because of exposure to increased amounts of ACTH. Symptoms that may appear soon after birth in neonates with classic CAH include dehydration, hypotension, low sodium (referred to as salt wasting ), arrhythmia, and shock (also known as: adrenal crisis). The extent of salt wasting may vary depending on the severity of aldosterone deficiency. In some cases, only cortisol synthesis is impaired and this is referred to as simple virilizing nonsalt wasting CAH . Female neonates with CAH may present with virilization and ambiguous genitalia with normal internal reproductive organs. This presentation is caused by excessive androgen production by the fetal adrenals secondary to increased ACTH drive.
The stage of gestation when the fetal adrenals begin producing physiologically relevant amounts of cortisol is not definitively known. Studies of steroidogenic enzyme expression in adrenal cortical cells suggest that the definitive zone gains the capacity to produce aldosterone, and the transitional zone produces cortisol by the middle of the third trimester. Interestingly, ambiguous and masculinized genitalia of female infants with CAH suggest exposure to excessive androgen in the form of DHEA, late in the first trimester when the urogenital sinus undergoes androgen-dependent differentiation. This implies that the fetal adrenals produce cortisol and exert negative feedback on pituitary ACTH secretion during the first trimester. Thus indirect data suggest that the fetal adrenals produce cortisol before 12 to 15 weeks, which then declines until 25 to 30 weeks’ gestation, after which transitional zone cells express steroidogenic enzymes needed for cortisol synthesis de novo from cholesterol. Mineralocorticoid production by the human fetal adrenal cortex is low early in gestation but increases during the third trimester. At term, 80% of the aldosterone in human and rhesus monkey fetal blood appears to originate from the fetal adrenal. In 18- to 21-week human fetal adrenals, the mineralocorticoid metabolic pathway localizes to the definitive zone, but its activity is low and unresponsive to secretagogues. Nonetheless, under normal conditions the steroidogenic capacity of the fetal adrenals to produce the cortisol and aldosterone is in place by the middle of the third trimester in preparation for the HPA axis to perform its vital function after birth.
Fetal Maturation and Parturition
At birth the fetus, as a newborn, must abruptly adapt to the extrauterine environment and independently establish and maintain physiological homeostasis. Success of this transition requires that organ systems needed for extrauterine life (such as lungs, gut, adrenals, thyroids, kidneys, liver, pancreas, immune system) are functionally mature before, or at least soon after, parturition. Maturation of the fetal lungs with the capacity for gas exchange is especially important. Cortisol is a key hormonal regulator of this process and promotes the functional maturation of multiple fetal organ systems before parturition. Interestingly, in some species, the cortisol responsible for fetal maturation also triggers parturition. For example, in sheep, activity of the fetal HPA axis increases during the week before parturition so that the fetal adrenals secrete a surge of cortisol that not only stimulates fetal organ maturation (especially surfactant production by the lungs) but also triggers parturition by inducing the metabolism of placental progesterone to androstenedione, leading to a systemic decrease in maternal progesterone levels that induces labor. Thus in the central role, the fetal HPA axis produces cortisol to ensure that fetal maturation precedes parturition and that the two processes are coordinated. The discovery that cortisol promotes fetal lung maturation led to the widespread clinical uses of synthetic glucocorticoids that cross the placenta to promote fetal lung maturation in women with threatened preterm birth. This therapy increases the survival rates of preterm infants mainly by decreasing the severity of respiratory distress syndrome because of pulmonary insufficiency in premature infants. Thus as with most species, glucocorticoid stimulates maturation of the human fetus, preparing it for life outside the uterus. However, unlike the sheep, neither glucocorticoid or activity of the fetal HPA axis induces human parturition.
In sheep, the cortisol needed to induce fetal maturation and trigger parturition originates in the fetus, whereas maternal cortisol or cortisol therapy to the maternal side only, has no effect. The reason for this finding is that the sheep placenta inactivates maternal cortisol by expressing the 11ß-HSD-2 enzyme that converts cortisol to the inactive cortisone. This is likely a safeguard to protect the developing fetal brain from adverse effects of glucocorticoid on neuronal development. Similarly, the human placenta prevents maternal cortisol from entering the fetal compartment throughout most of human pregnancy. This is why synthetic glucocorticoids that the placenta does not metabolize are used to treat women in preterm labor. This barrier, however, appears to collapse late in the third trimester. Evidence for this is that fetuses that have impaired cortisol production because of 21-hydroxylase deficiency are usually born at term without any apparent signs of organ immaturity. This suggests that another source of glucocorticoid, possibly from the maternal adrenals, could contribute to fetal organ maturation. If this is the case, maternal cortisol must cross the placenta. Late in human pregnancy, the placental barrier to maternal cortisol appears to weaken. The evidence for this is that estriol levels in the maternal circulation during late pregnancy are inversely related to the circadian changes in circulating maternal cortisol levels, suggesting that maternal cortisol crosses the placenta and exerts negative feedback on the fetal HPA axis, leading to a decrease in ACTH stimulation of DHEA-S production that, in turn, causes a decrease in placenta estriol production. Increased transfer of maternal cortisol to the fetus may represent a backup mechanism to ensure fetal lung maturation before term parturition.
The Hormonal Control of Human Parturition
The gravid uterus accommodates the growing conceptus physically and immunologically. For most of pregnancy the extracellular matrix of the uterine cervix is rigid and noncompliant, which effectively closes the uterine outlet. In contrast, the myometrium of the uterine wall relaxes, becoming compliant, growing mainly by cellular hypertrophy, and distending in response to the growing conceptus and amniotic fluid. The maternal immune system is also suppressed, especially at the maternal-fetal interface in the decidua, to prevent rejection of the semiallogenic fetal tissue. At parturition, the gravid uterus undergoes a series of temporally coordinated transformations, such that the myometrium becomes highly contractile and excitable and contracts rhythmically and forcefully to become the engine for birth, and the extracellular matrix of the cervix softens to allow dilation and opening of the gateway for birth. In addition, the fetal membranes weaken and rupture to release the fetus. The process also is associated with immune activation in the decidua that produces extensive tissue-level inflammation and the local production of prolabor uterotonic factors, such as prostaglandins. These events make up what we call labor , which is clinically defined as rhythmic and forceful contractions with softening and dilation of the cervix and rupture of the fetal membranes. The process culminates in the forceful expulsion of the fetus, placenta, and fetal membranes, including the decidua, and through hemostasis, involution, and endometrial regeneration, the uterus reverts to the nonpregnant state to resume menstrual cyclicity.
Hormones control the process and timing of parturition. Progesterone and estrogens affect the growth and contractility of the myometrium, the immunologic/inflammatory state of the decidua, and the mechanical integrity of the cervix. In this regard, the balance between the propregnancy actions of progesterone that dominate for most of pregnancy, and the prolabor actions of estrogens that occur as part of the parturition process, is critical. For most of pregnancy, progesterone and estrogen exert uterotropic effects to promote myometrial cell hypertrophy and increase blood flow to the uterus and cervix. Progesterone exerts a relaxatory and antiinflammatory effect on the uterine tissues to block labor by maintaining closure of the cervix and quiescence of the myometrium. At parturition, the effects of progesterone are lost and estrogens dominate to promote the expression of genes in myometrial and cervical cells whose products increase uterine contractility and cervical softening. Thus a general characteristic of viviparous species is that the withdrawal of the progesterone block triggers parturition, which permits stimulatory estrogenic actions to increase myometrial contractility and excitability and promote cervical softening and membrane weakening. Cortisol from fetal or maternal adrenals, does not appear to contribute to the hormone control of human parturition. Evidence for this is that parturition occurs normally at term in pregnancies where the fetus has CAH because of the inability to produce cortisol, and glucocorticoid therapy does not hasten the parturition process.
Placental CRH may be involved in the regulation of human parturition. Maternal plasma CRH concentrations—reflecting CRH mainly of placental origin—increase exponentially with advancing gestation, and the rate of change as gestation progresses is predictive of the gestation length. The more rapid the increase in maternal CRH from midgestation, the earlier parturition occurs. A slower rate of increase is associated with postterm birth. The physiology and molecular biology underlying this association is not understood. In vitro experiments suggest some possibilities. CRH receptors have been identified in myometrial cells and fetal membranes. CRH stimulates PG secretion from decidua and amnion, and increases myometrial contractility by augmenting responsiveness to oxytocin and PGF 2 α. Paradoxically, placental CRH increases adenylate cyclase activity in myometrial cells, which promotes relaxation, but this effect is lost late in pregnancy, possibly because of changes in CRH receptor isoforms in myometrial cells.
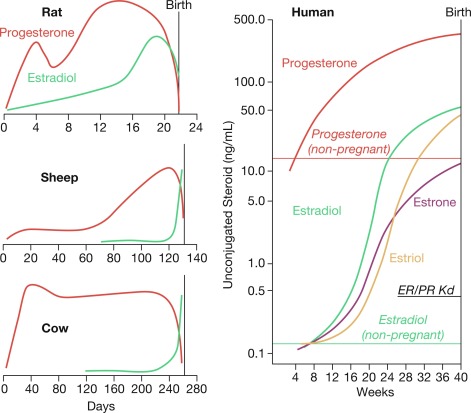
How progesterone withdrawal happens to achieve parturition differs between species, depending on the source of progesterone that maintains pregnancy. For example, in rodents the maternal CL produces progesterone during pregnancy and CL regression triggers parturition, leading to systemic progesterone withdrawal. In sheep, the placenta exclusively produces progesterone and its withdrawal is induced by a prepartum cortisol surge that induces the expression of the 17α-hydroxylase enzyme in placental cells that diverts progesterone from secretion to conversion to androstenedione, which is converted to estradiol. This enzymatic induction leads to a systemic decrease in maternal progesterone levels and a concomitant increase in estrogen levels that triggers parturition. In contrast to rodents and sheep, parturition in women occurs without a systemic progesterone withdrawal ( Fig. 5.4 ). Instead, the gravid uterus receives high levels of progesterone and estrogens—because of activity of the fetoplacental unit—for most of pregnancy and during labor and delivery. Although this suggests that progesterone withdrawal does not trigger human parturition, clinical studies show that disruption of progesterone signaling with agents, such as mifepristone, a selective progesterone receptor modulator/antagonist, increases myometrial contractility, and induces labor at all stages of pregnancy. That observation suggests that a functional, rather than systemic, progesterone withdrawal triggers human parturition, whereby uterine cells become refractory to progesterone.