Fig. 8.1
ASBMR-esta blished AFF criteria and schematic with radiograph illustrating the major features of AFFs
The incidence of AFFs is very low [8], especially when compared to the number of osteoporotic fractures prevented by bisphosphonates and other antiresorptive therapies [9, 10]. The relative risk (or odds ratio) of AFFs is more variable and has been reported as ranging from 2 to 47 [11]. Factors contributing to the variability of the relative risk are important to consider: does the variability just reflect regional variation, specifically the relative length of time patients in different geographic areas have been on antiresorptive drugs or, as recently suggested [12], variations in the authors’ definitions of an atypical fracture? Since the calculation of relative risk depends on the types of patients in the case and control groups, the latter is likely the case. In a letter to the editor of Journal of Bone and Mineral Research that was published in Acta Orthopaedica [12] explaining the variation in observed AFF rate in different studies, the authors of the letter pointed out that the “fatigue-type” fracture noted in elderly populations and associated with material failure was referred to as atypical whether or not the fracture occurred in the femoral shaft or in the subtrochanteric region. Hence, AFFs reported in some studies did not meet the ASBMR definition. Factors contributing to the discrepancy in odds ratio could include the absence of radiographic data, a broad definition of shaft fractures, and the absence of a fracture line perpendic ular to the cortex [13].
Conditions and Treatments Associated with AFFs
Independent of the relative risk of AFF, the majority of existing studies that based their evaluation of AFFs on the ASBMR criteria found an association between duration of use of bisphosphonates and incidence of AFF [8, 14–16], with incidence increasing with prolonged use of antiresorptive drugs. Association of bisphosphonates with AFF, however, remains debatable as AFFs continue to be reported in bisphosphonate-free patients [17]. Large population-based studies of older women, with validated fracture codes, support the association of long-term bisphosphonate (BP) use and AFFs [18]. In contrast, based on propensity data, AFFs were equally common to people using bisphosphonates and to those using raloxifene (a selective estrogen receptor modulator) or calcitonin [19]. Notably, reports of subtrochanteric (atypical) fractures began after the introduction of bisphosphonates [20]. This observation, and the radiographic findings indicating the association of AFF with long-term (>5 years) bisphosphonate use [21, 22], lends support to the association between AFFs and antiresorptive drugs, but the data to date have not been conclusive. Readers are again reminded that today, bisphosphonates and other antiresorptive drugs have been remarkably effective in reducing osteoporotic fracture incidence [3, 8–10].
The question to be addressed in this review is why the use of antiresorptive medication results in AFFs. Although this fracture is a rare occurrence, the etiology is important. Possible answers to the association between bisphosphonate treatment and AFF include (1) patients who get AFFs did not need bisphosphonates or related drugs but were treated, resulting in a situation in which bone remodeling is oversuppressed; (2) oversuppression alters the material properties of bone to such an extent that the tissue becomes more brittle; (3) alterations in bone morphology increase the stresses on the femur and put specific sets of patients at risk; and (4) the patients were receiving other medications, such as glucocorticoids (GC) , that also affect the bone tissue properties, and the combined treatment is adverse. Each of these possibilities is supported by existing data as discussed below, and finally, some component of each of these factors likely contributes to the development of AFFs. One key issue in looking at both etiology and mechanism is the variability in the profiles of the small number of AFF patients.
Serum and Other Noninvasive Clinical Markers of AFFs
Serum or other noninvasive clinical markers would be desirable to detect early signs of AFFs or to recognize individuals at risk for AFFs who should not be treated with antiresorptive drugs associated with AFFs. To date, unfortunately, no definitive associations have been identified between AFF and serum markers. However, through these studies, other metabolic contributors to AFFs have been identifi ed (see section “Contribution of Metabolic Disease to Development of AFF”) [23, 24].
Histological Markers of AFF
Studies examining bone tissue next to the fracture site generally found decreased bone formation but normal bone remodeling and no evidence of a mineralization defect in patients with AFFs. Because these tissues were collected at variable times after the fracture occurred and did not always include double labeling to measure bone formation rate, the meaning of the overall histomorphometric data is difficult to interpret. The presence of a fracture callus and radiolucency on the lateral cortex where these fractures initiate suggests that bone tissue is still actively formed and resorbed. However, a limited number of case reports of AFFs show no or very few double labels indicating that mineralization is not occurring [25].
Are AFFs “Stress” Fractures Rather than “Insufficiency” Fractures?
Insufficiency fracture
Insufficiency fracture is a fracture that occurs at a load that would normally not cause failure, because the mechanical strength of the bone is compromised. The same load applied to the skeleton of a healthy individual would not result in a fracture.
Stress fractures are fractures which occur as a result of repetitive loading at subfailure loads in the skeleton of a healthy individual.
Several features of AFFs suggest that these failures result from repetitive loading (fatigue-type fractures). AFFs occur with minimal or low loads. Fracture occurs when the applied loads exceed the load-bearing capacity of a structure such as a long bone. This process can result either due to a single high overload (traumatic failure) or as a result of repeated subfailure loads (fatigue failure). Fatigue failure results from cyclic loading over time at loads that are below the single fracture load, which appears to correspond to the AFF mechanism. Repetitive loading in fatigue initiates damage in the form of cracks or microcracks, damage accumulates with continued loading until these cracks propagate and coalesce to produce catastrophic structural failure. This process of damage development and propagation depends on cortical geometry and tissue mechanical properties. Therefore, AFFs likely result from a fatigue-based mechanism.
A further indication that AFFs are stress fractures is the presence of a localized periosteal response and the prodromal symptoms. The periosteal response includes not only a general thickening of the cortex but also the “beaking” seen on the lateral cortex and is often accompanied by a radiolucent line that is presumably a localized healing or remodeling response. Similar local tissue responses are present in stress fractures induced through strenuous athletic activity. The cortices of bones of patients with AFFs appear thicker, but whether this reflects bisphosphonate treatment or AFF development or a combination of the two is not known.
If AFFs are indeed stress fractures , then two bone tissue properties are critical to characterizing the tissue-level changes: fatigue behavior for understanding the performance under repetitive, non-failure loading, and fracture toughness for understanding crack propagation. While these properties have been reported for healthy human cortical bone tissue, our knowledge of the effects with bisphosphonate treatment and remodeling suppression is limited.
Factors Contributing to AFFs
As mentioned above, a variety of causes may contribute to the development of AFFs. Conceptually, these factors that may underlie AFF can be considered in three broad categories. First, the use of antiresorptive drugs , particularly bisphosphonates, can lead to oversuppression of remodeling and result in alterations in bone material properties that adversely affect the mechanical behavior of the femur and the properties of cortical bone tissue (Fig. 8.2). Alterations in cancellous bone with bisphosphonate treatment that contribute to reductions in typical osteoporotic fractures will not be addressed here. Second, individual variations in skeletal morphology could contribute to the presence of high stresses in femoral cortex, a location that bears high loads and normally does not fracture. Finally, as introduced above, the presence of underlying metabolic disease likely also contributes. Here, we review the data supporting each of these mechanisms and the impact on the ability of the femur to bear functional loads.
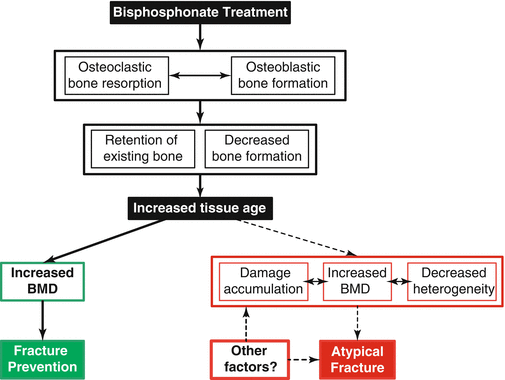

Fig. 8.2
Illustration of the possible actions of bisphosphonates leading to either reduced typical fracture rates (primary pathway leading to green box) or to altered material properties in patients with AFFs (secondary pathway leading to red box). Bisphosphonate treatment inhibits the activity of osteoclasts, disrupts the coupling between osteoblasts and osteoclasts, and, to a lesser extent, inhibits osteoblastic action
BP-Induced Remodeling Suppression Leads to Adverse Tissue Material Changes
Bisphosphonates are administered to reduce bone turnover in individuals with osteoporosis. Therefore, impaired bone turnover is a likely suspect as a cause underlying AFFs. However, the histological evidence is limited and mixed, based on double labeling of tissue to indicated active bone remodeling. AFF patients given dual tetracycline labels prior to biopsy have been reported to show only single or no labels [25, 26], while well-defined double labels have been reported in a patient on long-term bisphosphonate treatment (>9 years) [27]. Thus, oversuppression of bone remodeling may not be the sole cause of AFFs . This suppression of bone turnover may contribute to multiple material changes including increased bone mineral content of the tissue, reduced tissue heterogeneity, and increased microdamage formation (Fig. 8.3). These individual material changes can combine to lead to brittle failure of the tissue and whole bone.
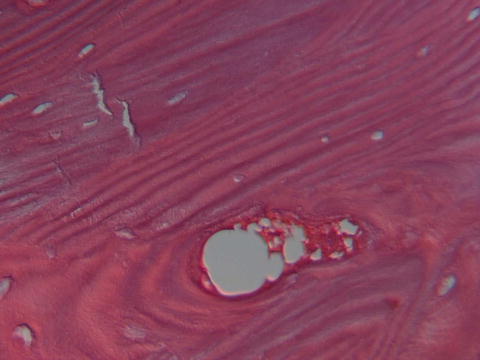

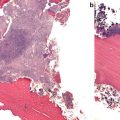
Fig. 8.3
Photomicrograph showing multiple cracks in a hematoxylin- and eosin-stained biopsy of a female patient with osteoporosis treated for 6 years with alendronate who sustained an atypical femoral fracture. Courtesy of Dr. M. Klein, Department of Pathology, HSS
Increased Bone Mineral Content
Increased bone mineral content of bone tissue is a positive outcome of reduced turnover rates and the primary reason osteoporotic individuals are treated with bisphosphonates. However, this positive effect has limitations that may contribute to AFF development in the small cohort of individuals who develop these fractures. In particular, this increase in mineral content is accompanied by an increase in the mean age of the tissue. The absence of remodeling produces not only a greater volume of mineralized tissue but also a reduced volume of newer younger tissue, resulting a more homogeneous tissu e with an increased degree of min eralization.
Reduced Tissue Heterogeneity
BP treatment has multiple effects on tissue composition and ultimately results in a more homogenous tissue without the heterogeneity in composition that is normally a hallmark of bone tissue. Changes in bone composition have also been reported both in short-term iliac crest biopsies from alendronate-treated women [28], iliac crest biopsies from individuals with AFF on bisphosphonates [25], and in biopsies obtained adjacent to the fracture site in bisphosphonate-treated women [25, 29]. Compositional variability was reduced in biopsies from individuals with AFFs [25, 29]. When mineral composition was examined as a function of typical or atypical fracture morphology in patients on bisphosphonates, the compositional properties of tissue from patients with AFFs (n = 6) fell within the range of values from patients with typical fractures (n = 14), except the mean cortical degree of mineralization was 8 % greater in AFF tissue (atypical 5.6 ± 0.3 versus typical 5.2 ± 0.5) than in bisphosphonate-treated patients with typical osteoporotic fractures [29]. Biopsies were also included from bisphosphonate-naïve individuals with fragility fractures, none of whom experienced AFFs. Although the mean values of most compositional properties were similar in both fracture groups, the tissue in bisphosphonate-treated patients had a more uniform composition than that of bisphosphonate-naïve patients with typical fractures. A study of iliac crest biopsies from AFF and control patients focused on trabecular tissue and examined similar compositional outcome measures [25]. While AFFs were not only present with bisphosphonate treatment, the biopsies were obtained from four patients on long-term bisphosphonate therapy. Trabecular tissue from the iliac crest of individuals with AFF had increased degree of mineralization, increased collagen maturity, and decreased mineralization heterogeneity. These compositional and morphological features could explain the higher incidence of fracture in these patients. Similar decreases in bone material heterogeneity were reported with treatment by different bisphosphonates including alendronate [28–32], risedronate [33], and zoledronic acid [34]. Oversuppression of remodeling by long-term treatment with bisphosphonates allows the proliferation of microcracks that weaken the bone tissue. Thus, loss of heterogeneity may reflect suppressed bone remodeling and inability to repair microcracks while also resulting in less resistance to crack formation and propagation.
Increased Collagen Cross-Linking
A further material change with bisphosphonate treatment is increased nonenzymatic collagen cross-links [28, 29]. At the tissue level, reductions in post-yield toughness were associated with increased nonenzymatic collagen glycation in cortical tissue of the tibia from dogs treated with high doses of alendronate, but not when clinically equivalent doses were administered [35]. While limited data exist for the composition of bone in individuals with AFF, changes in the collagen maturity, a measure of the ratio of nonreducible to reducible collagen cross-links, were reported in both cortical and cancellous tissue [25, 29] and suggest that they may arise from prolonged bisphosphonate treatment.
Increased Microdamage Formation
Suppression of remodeling by bisphosphonates increases microdamage in cortical bone tissue. Bone microdamage increases due to diminished repair [36–39] and increased crack burden, possibly due to a less heterogeneous tissue, leading to failure at lower energy and in a more “brittle” mode. Reduced post-yield toughness of bone tissue was associated with increased crack lengths and density in dogs treated with high doses of either alendronate or risedronate [40, 41]; however, increased microdamage was not present in animals treated with etidronate [42]. As described above, remodeling suppression reduces or eliminates “normal” bone tissue microstructural heterogeneity that results from having osteons and cement lines of different ages. In healthy tissue, the local differences in material properties produced by microstructural features are essential in dissipating energy and blunting crack propagation. Loss of these natural interfaces and crack-blunting processes can result in brittle-mode-type fractures [36, 37, 42, 43]. In addition, the lack of remodeling limits the repair of this damage.
Both brittleness and loss of heterogeneity allow greater progression of microscopic cracks (Fig. 8.2) that can occur with usual physical activity. Material heterogeneity is a mechanism that normally dissipates crack tip growth energy, thereby limiting crack growth. In a more homogeneous tissue, the energy to grow a crack is reduced and crack progression is less impeded. Targeted repair of cracks by newly activated BMUs appears to be preferentially suppressed by BPs [38]. In classical fracture mechanics, loss of material heterogeneity is associated with increased crack initiation and less resistance to crack propagation, leading to a greater risk of fracture [44, 45]. In cortical bone, transverse cracks are normally deflected longitudinally, limiting the effects of damage when the tissue is loaded. The remarkable straight transverse fracture line seen with AFF is an indicator of the dramatically altered tissue material pro perties and the failure of usual mechanisms to bridge or deflect the crack.
Decreased Toughness of Cortical Bone
In cortical bone, the functional outcome of these multiple effects of BP treatment can be to alter the mechanical behavior of bulk samples as in the case of AFF, presumably reflecting the combined effects of the increased bone mineral content, reduced heterogeneity, and increased microdamage associated with suppressed bone turnover. In general, bone material strength and stiffness were not altered in cortical bone, but post-yield toughness decreased at high doses [40, 46]. Preclinical studies examining bone properties primarily have been performed in estrogen-replete dog models using supraphysiological BP doses [37]. The majority of these studies have examined alendronate treatment, but these changes are also reported with risedronate and etidronate. The reduced post-yield deformation likely reflects increased damage formation, contributing to the brittle failure evident in AFF. Excessively reduced post-yield toughness produces brittle behavior, which is defined as the abse nce of post-yield deforma tion.
Nanomechanical Behavior
When the mechanical behavior is exam ined in small volumes of cortical bone tissue, the elastic behavior has been reported to be both reduced and unaffected. The reported differences may reflect levels of scale. Reference point indentation (RPI) has previously shown differences in the in vivo bone microindentation properties at the anterior tibial cortex of patients with hip fractures compared to age-matched controls [47]. Using RPI, no differences were present among typical and atypical fracture cases at the mid-tibia for microindentation properties nor were these cases different from patients on long-term bisphosphonate treatment, whose values were intermediate between controls and those who sustained fractures [48]. The similarity of properties among fracture cases suggests that the alterations in tissue-level material properties in individuals with AFF are similar to those of individuals who sustain osteoporotic fractures. Micromechanical properties were reduced in patients using alendronate for 6–10 years, corresponding with decreased mineral crystallinity, elastic modulus, and contact microhardness [49]. Tissue from patients with AFF was not examined. These relatively larger sampled volumes may include damage and other effects, but these effects would also be present with RPI, so in vivo measurement may be a critical difference. Nanomechanical analysis of iliac crest biopsies of individuals with severely suppressed bone turnover and atypical fractures (SSBT) showed no differences in cortical modulus or hardness of cortical tissue from AFF patients relative to age-matched and young female controls and osteoporotic individuals who had experienced vertebral fractures [50]. Plastic deformation resistance was greater in tissue from individuals with SSBT. Nanomechanical differences were present in their cancellous tissue. Tissue-level heterogeneity of the elastic modulus and plastic deformation resistance was reduced in the cortical bone of the bio psies from patients with suppressed bone turnover. While hardness and plastic deformation resistance are inelastic measures, their relationship to the tissue-level toughness has not been established.
Finally, if AFFs are due to impaired remodeling and associated mechanisms, as described, a similar fracture pattern might be expected in individuals with pycnodysostosis, a rare disorder with mutations in cathepsin K, the enzyme that digests the organic matrix of bone during the remodeling process [51]. In fact, AFFs have been reported in some patients with pycnodysostosis [52]. However, AFFs have not been reported in other cases of pycnodysostosis or in patie nts with other defects in remodeling, such as osteopetrosis.
Lower Limb Morphology Alters Stresses in the Femur
The frequent bilateral incidence of AFF suggests a mechanical etiology associated with individual anatomy, in addition to any remodeling-induced changes. Changes in lower limb skeletal geometry , such as femoral neck-shaft angle and femoral curvature [53], will alter the stresses and strains experienced in the femoral diaphysis with loading. Skeletal structure and kinematics have been correlated with the risk of stress fracture in young active individuals [54–56]. The incidence of typical osteoporotic hip fractures is lower in Asian women [57], yet the incidence of AFF is higher [22, 58]. Femoral geometry differs between Asian and Caucasian women, including shorter hip axis lengths and smaller femoral neck-shaft angles in Asian women [59]. If such geometric variations contribute to typical fracture rate differences, similar factors may also explain AFF incidence rates. The exact contribution of lower limb skeletal morphology to AFFs is yet to be determined, but the eviden ce shows morphology is likely a contributing factor [60].
Contribution of Metabolic Disease to Development of AFF
In addition to osteoporosis, other metabolic abnormalities may be present in AFF patients. Comorbidities of AFFs such as bisphosphonate therapy, use of GCs, and other complications likely contribute to the alterations in tissue properties that result in AFFs. In a review of 31 published cases and one unpublished case, proton pump inhibitor and GC use were found in a majority of the AFF patients [24]. Moreover, ~76 % of the AFF patients had at least one major chronic disorder. AFFs occurred in patients with hypophosphatemia, indicating that some underlying disorder in metabolic status was a contributing factor to AFFs. A recent study using ASBMR criteria to identify AFFs examined serum markers in an Italian population comparing women with AFF (n = 11) to women with typical fractures (n = 58) admitted to a single hospital over a period of 3 years [23]. Younger age, use of bisphosphonates, and hypercalcemia were features of the AFF patients, while elevated PTH was reported to be protective. The younger age of the patients is supported by other studies [24, 61]; however, hypercalcemia, earlier menopause, and higher BMI associated with AFFs have not been confirmed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree