Neoplastic
Cysts
Inflammatory/infiltrative
Vascular
Pituitary adenoma
Craniopharyngioma
Chordoma
Metastasis
Meningioma
Germ cell tumor
Glioma
Granular cell tumor (pituicytoma)
Hypothalamic neuronal hamartoma
Dermoid
Pituitary carcinoma
Rathke’s cleft cyst
Arachnoid cyst
Lymphocytic hypophysitis
Granulomatous hypophysitis
Sarcoidosis
Langerhans cell histiocytosis
Eosinophilic infiltration (Churg–Strauss)
Infection: bacterial, mycobacterial, fungal, protozoal
Carotid aneurysm
Pituitary adenomas are arbitrarily categorized by size as microadenomas (<10 mm) or macroadenomas (≥10 mm). On high-resolution CT, pituitary adenomas are typically hypodense compared with the normal gland on both unenhanced and contrast-enhanced images. On MRI imaging [6] 80–90 % of microadenomas appear as a focal hypointense lesion compared with the normal gland (Fig. 5.1) on unenhanced T1-weighted images. After gadolinium, an adenoma typically enhances less avidly than the rest of the gland (Fig. 5.2a). On T2-weighted images up to 50 % of microadenomas are hyperintense. Other findings that can be seen with pituitary adenomas are focal erosion of the sella floor or focal convexity of the superior surface of the gland. Deviation of the pituitary stalk can be seen with microadenomas but may simply represent normal variation. Macroadenomas have similar signal characteristics as microadenomas. Comparing the pre and post contrast signal to the normal pituitary may not be possible as the normal pituitary may be compressed and totally obscured by the macroadenoma. Macroadenomas may grow and extend outside of the confines of the sella turcica. Superior extension of the tumor can cause displacement or compression of the optic nerves and chiasm (Fig. 5.2b). Some adenomas may grow inferiorly, erode through the floor of the sella and fill the sphenoid sinus. Adenomas that invade laterally into the cavernous sinus are unlikely to be cured surgically. Unfortunately both CT and MRI are not highly accurate in predicting cavernous sinus invasion. Encasement of the internal carotid artery is conclusive evidence. A tumor extending beyond the lateral aspect of the internal carotid artery on coronal MRI images is highly suggestive of cavernous sinus involvement. There are cystic variants of pituitary adenomas and cystic degeneration and hemorrhage may also be present.
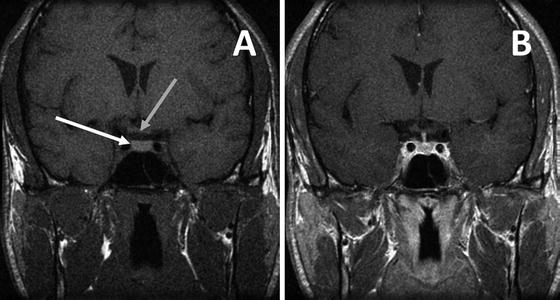
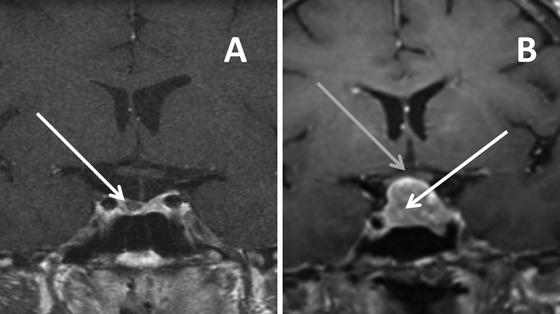

Fig. 5.1
Normal pituitary gland on MRI T-1 weighted image in the coronal plane prior to contrast (a) showing the normal pituitary gland (white arrow) and the relationship to the optic chiasm (gray arrow). T-1 weighted image after gadolinium (b) demonstrates uniform enhancement of the normal pituitary gland and infundibulum

Fig. 5.2
MRI T-1 weighted images in the coronal plane after gadolinium showing: (a) a pituitary microadenoma (white arrow) located in the right side of the gland that enhances less than the normal pituitary tissue and (b) a pituitary macroadenoma (white arrow) with suprasellar extension displacing and deforming the optic chiasm (gray arrow)
The imaging characteristics of Rathke’s cleft cysts [6] reflect their etiology. These non-neoplastic cysts arise from remnants of epithelium from Rathke’s pouch. They are typically seen in the center of the gland, although they can be laterally located or present in the suprasellar space (Fig. 5.3). Many of these cysts are isointense with cerebral spinal fluid (CSF) on MRI imaging, however, if they contain proteinaceous fluid they may be hyperintense on T1- and T2-weighted sequences. They lack contrast enhancement and do not contain calcifications. Occasionally a Rathke’s cleft cyst can enlarge and compress the optic chiasm.
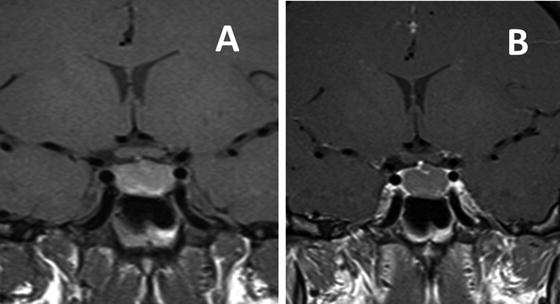

Fig. 5.3
MRI T-1 weighted images in the coronal plane showing an intrasellar Rathke’s cleft cyst. Prior to contrast (a) the cyst shows mild increased intensity compared to normal brain. After gadolinium (b) the cyst does not enhance
History, Physical Examination, and Laboratory Findings
Symptoms, findings on physical examination and laboratory abnormalities associated with pituitary tumors are due to the effects of hormonal excess, hormonal deficiency or local effects the mass on surrounding tissues. Detailed questioning regarding the symptoms, and examination for the physical signs associated with excess and deficiency of each hormonal axis should be obtained. Symptoms related to mass effect include vision loss which is classically loss of peripheral vision from compression of the optic chiasm. Other patterns of visual loss can occur due to anatomic variation in the anatomy of the optic nerves and chiasm. As vision loss can occur very slowly over years some patients may not be aware of vision loss even in the presence of significant visual field deficits. Although loss of peripheral vision may be demonstrable on physical examination, automated perimetry should be obtained with masses that approach or contact the optic apparatus. Headaches are unlikely to occur with small masses that are confined to the sella. Larger masses with extrasellar extension can cause headaches. Interestingly, likely due to slow growth and tissue adaptation, many patients with huge sellar based masses do not have headaches. The sudden onset of a severe headache, acute onset of symptoms of pituitary insufficiency and cranial nerve deficiency such as diplopia or ptosis can be seen with hemorrhage in a pituitary adenoma (pituitary apoplexy). Polyuria and polydipsia will be present in lesions affecting the posterior pituitary, pituitary stalk or hypothalamus that cause deficiency of antidiuretic hormone (diabetes insipidus). Although it is uncommon, many different systemic illnesses can involve the pituitary and parasellar area and a thorough review of systems should be completed. If the history or physical exam suggests hormonal excess or deficiency the appropriate hormonal laboratory evaluation should be obtained including dynamic testing if necessary. The laboratory evaluation for asymptomatic patients with a pituitary mass is discussed below.
Evaluation of the Incidentally Found Pituitary Mass
Clinicians are commonly presented with patients who have had a pituitary mass incidentally discovered. The “pituitary incidentaloma” is defined as an asymptomatic mass in the pituitary, found on imaging done for an unrelated reason. The majority of these lesions are small, usually less than 1 cm in diameter, and represents either pituitary adenomas or Rathke’s cleft cysts. To decide what tests, if any, should be obtained and if treatment or observation is indicated, one needs to confirm the patient is asymptomatic and also consider the potential for, and the clinical impact of, hormone deficiency, hormone excess, and tumor growth.
Incidentally found macroadenomas are commonly associated with hormone deficiencies with a reported prevalence of hypopituitarism ranging between 15 and 57 % [7, 8]. The presence of hormone deficiencies in incidentally found microadenomas is controversial, likely reflecting the arbitrary size cutoff of greater or less than 1 cm. and the fact that subtle deficiencies may be present despite normal baseline hormonal levels. Retrospective studies suggest that incidentally found microadenomas have a very low chance of being associated with hormonal hypofunction with most studies reporting a 0 % incidence. However, this needs to be interpreted in light of a study [9] involving 38 patients with microadenomas (55 % incidentally found, 45 % found on imagining done to evaluate abnormal laboratory tests suggesting pituitary dysfunction). GH releasing hormone (GHRH)/arginine stimulation found 50 % of these patients to be GH deficient. In addition, basal hormone levels and/or 1 μg Cortrosyn stimulation identified at least one deficiency of gonadotropins, TSH, and/or ACTH in 50 % of the patients. This led the Endocrine Society task force that developed the clinical guidelines for pituitary incidentalomas to favor screening for hormone deficiency in tumors greater than 5 mm [5].
Recommendations vary widely as to the specific laboratory tests that should be obtained. Given the current literature, laboratory testing to identify subclinical hypopituitarism in asymptomatic patients with an incidentally found pituitary mass should be obtained when the mass is greater than 5 mm in size (Table 5.2). It is reasonable to obtain TSH and free thyroxine to rule out secondary hypothyroidism, IGF-1 to screen for GH deficiency, and a morning cortisol level to screen for secondary adrenal insufficiency, realizing that basal levels of IGF-1 and morning cortisol may not be adequate to determine normalcy or deficiency and dynamic testing may be required. Obtaining a testosterone level in men and a menstrual history in women will determine if hypogonadism is present. Measuring LH and FSH may be helpful in some instances, for example if there is concern about coincidental primary hypogonadism.
Table 5.2
Recommended hormonal tests for asymptomatic patients with an incidentally found pituitary mass
To rule out subclinical hormone excess | To rule out subclinical hormone deficiency | ||
|---|---|---|---|
All sizes | Size ≤5 mm | Size >5 mm | |
Prolactina | X | ||
IGF-1b | X | X | |
FT4, TSH | X | ||
Testosterone (men) | X | ||
Menstrual history (premenopausal women) | |||
AM Cortisolb | X | ||
The most common hormone overproduced in incidentally found pituitary masses is PRL with an incidence of 12–28 % [8, 10, 11]. Prolactin measurement should be done after serial dilutions of the serum in macroadenomas as falsely low values may be present when prolactin concentration is are in fact very high due to the high dose hook effect of the assay [12]. Prolactinomas have potential for morbidity, testing is easy, and safe and effective treatment is available.
The incidence of GH overproduction by an incidentally found mass is between 2 and 8 % [8]. In early cases symptoms and physical findings may be quite subtle, but there is potential for serious morbidity and mortality if GH excess is not detected. There is also a high likelihood of surgical cure when the tumor is small.
No instances of ACTH excess have been reported in clinical studies of pituitary incidentaloma. Autopsy studies report between 1 and 13.8 % of the adenomas stained for ACTH [13]. The screening tests for ACTH excess are cumbersome to perform and, particularly in asymptomatic patients, have high false positive rates [14]. Although Cushing’s disease is serious, screening for ACTH excess in patients with no clinical suspicion of glucocorticoid excess is not recommended due to the low prevalence and the high false positive rate of the screening tests [5].
Although autopsy studies indicate 4 % of incidentally found adenomas stain for gonadotropins, LH and FSH are usually in the normal range and are often biologically inactive in surgically proven gonadotropin adenomas. Rare cases of elevated testosterone levels in men and ovarian hyperstimulation in women due to gonadotropin secreting pituitary adenomas have been reported [15, 16]. If a clinical syndrome is present with a gonadotropin producing adenoma it is usually hypogonadism associated with a macroadenoma.
TSH secreting pituitary adenomas are exceedingly rare, and none have been reported in clinical or autopsy series of incidentally found masses. These usually present clinically as macroadenomas with symptoms of hyperthyroidism [17]. Routine screening in the setting of a pituitary incidentaloma is not recommended.
Given the frequency and the clinical impact of over production of the various hormones, and the sensitivity and specificity of the screening tests, it is reasonable to obtain levels of PRL and IGF-1 to rule out subclinical excess secretion for all truly asymptomatic patients with incidentally found pituitary masses (Table 5.2).
An automated visual field examination should also be obtained at baseline if a macroadenoma is approaching or contacting the optic chiasm on MRI images [5]. This serves to determine if there is subclinical vision loss and also to serve as a baseline to determine if future growth, or surgical or radiation treatment, caused new vision loss.
Both microadenomas and macroadenomas have the potential to increase in size over time. Growth may occur after several years of stability. Macroadenomas likely grow more often (7–51 %) than microadenomas (0–14 %) [3]. Any increase in size of a macroadenoma has a higher chance of causing clinically significant mass effects.
Observation is appropriate if there is no hormonal over or under production, and the mass is not causing or threatening vision loss. If the decision is made to observe, repeat imaging with MRI scanning should be done initially at 6–12 months, and then annually for 2–4 years, and periodically thereafter. Doubling the interval since the last scan if no change is noted is appropriate. With macroadenomas, follow-up should include hormonal assessment for hypopituitarism, assessment for symptoms of mass effect and imaging with MRI. Since the decision to do surgery on a nonfunctioning macroadenoma is going to rest primarily on the development of vision loss, formal visual fields (if the mass is in proximity to the optic chiasm) should be obtained at these same intervals. All follow-up scans should be compared to the baseline scan in addition to the prior scan, since minor consecutive increases in size may not be appreciated.
Differential Diagnosis
A wide spectrum of lesions with various etiologies can manifest as masses in the sellar or parasellar region (Table 5.1). Excluding pituitary adenomas and Rathke’s cleft cysts, they represent only about 6 % of clinically apparent lesions [4]. Brief discussions of some of these lesions follow.
Craniopharyngiomas are tumors of epithelial origin that can affect both children and adults. Craniopharyngiomas are often located in the suprasellar space but can be within the sella. They can appear solid on imaging but often contain both solid and cystic components. Calcifications, seen on plain X-ray or CT, may be present. These tumors commonly present with vision loss, anterior pituitary hormone deficiencies, and diabetes insipidus [18].
Chordoma is an aggressive, rare bone cancer that is locally invasive, and has a predilection for the axial skeleton, with the most common sites being the sacrum, skull base, and spine. Parasellar chordomas usually involve the dorsum sella, clivus, or nasopharynx and cause local bone destruction (best seen on CT). Symptoms of headache or neck pain are common and diplopia or facial numbness can occur if cavernous sinus invasion is present [19].
Germ cell tumors can affect the central nervous system. The pineal gland is the most common site for intracranial germ cell tumors. These tumors can also be located in the suprasellar region, basal ganglia, posterior fossa, pituitary gland, or medulla. They may manifest as multiple discreet lesions and leptomeningeal spread occurs in 10–15 % of cases. Common symptoms include headache, diplopia, hypopituitarism, and diabetes insipidus [20]. They typically present in teenagers or young adults (peak incidence age 10–14 years) and rarely present in patients greater than 30 years old. The diagnosis can be facilitated by measuring the tumor markers beta-human chorionic gonadotropin (βHCG) and alpha-fetoprotein (AFP), which may be present in blood or CSF.
Metastatic tumors to the sellar area are usually asymptomatic from the pituitary standpoint and typically are found in patients with known metastatic disease. The posterior lobe of the pituitary or the hypothalamus are more commonly involved making diabetes insipidus the most common pituitary related symptom. Metastases may also cause anterior pituitary dysfunction, vision loss, diplopia, and retro-orbital pain. The most common primary sites are breast, lung, gastrointestinal tract, kidney, prostate, and melanoma [21].
Primary brain tumors may present in or around the sella. Parasellar meningiomas are often located by dorsum sella or clivus [22]. Radiologically meningiomas are typically isointense to hypointense to gray matter on T1-weighted MRI sequences and isointense to hyperintense on T2-weighted MRI sequences. They typically display intense homogeneous enhancement and may show calcification. Gliomas located in the parasellar region often arise from the optic nerves or optic chiasm and have an unpredictable clinical course [23].
Lymphocytic hypophysitis is a presumed autoimmune inflammation of the pituitary which is more common in premenopausal women (80–90 %), often presenting during pregnancy or postpartum, but can occur in men, children, and the elderly [24]. Recently it has been reported as a side effect of ipilimumab, an anticancer monoclonal antibody that stimulates cytotoxic T-lymphocytes [25]. Symptoms commonly include headache which is often intense. Pituitary insufficiency may involve only one hormone axis, including diabetes insipidus, or multiple hormone deficiencies may be present. Imaging can be variable showing diffuse pituitary enlargement, enlargement of both the pituitary and pituitary stalk, stalk enlargement only which may appear as a suprasellar mass. Usually homogeneous contrast enhancement is present. Occasionally a presumptive diagnosis of lymphocytic hypophysitis is made with a normal appearance of the pituitary on imaging.
Neurosarcoidosis can affect the parasellar region and often involves the hypothalamus and/or pituitary stalk [26]. Angiotensin converting enzyme (ACE) levels usually are elevated in the blood or CSF. Other organs are typically affected at some point in the course of the disease, but parasellar neurosarcoidosis can be the only manifestation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






