Evaluation of Eosinophilia
Robert M. Zemble
Andrea J. Apter
Eosinophilia is defined in this chapter as the presence of excess numbers of eosinophils in the blood or tissues. The upper limit of normal number of eosinophils in the circulation has been variably described as approximately 400 cells/μL (1–3). Eosinophilia is associated with allergic, infectious, neoplastic, or idiopathic disorders. This chapter focuses on the diagnosis and management of disease characterized by eosinophilia.
Eosinophils in the Blood
Wharton Jones is believed to have been the first scientist to recognize the eosinophil in unstained preparations of peripheral blood in 1876. Paul Ehrlich gave the cell the name eosinophil in 1879 because of the intense staining of its granules with acidic aniline dyes like eosin. The staining procedures he developed allowed the cell to be recognized and studied (4).
The eosinophil count can be estimated by multiplying the percentage of eosinophils from the differential white blood cell count by the total number of white blood cells. Normally, 1% to 5% of blood leukocytes are eosinophils (2,5). A manual differential should be obtained if eosinophilia is suspected. Various conditions may influence the eosinophil count. In patients with leukopenia, the percentages of eosinophils may be increased, but not their absolute number. This has been called pseudoeosinophilia (5). The number of eosinophils in the blood has a diurnal variation, being highest at night and falling in the morning when endogenous glucocorticoid levels increase (5). Exogenous glucocorticoids, endogenous glucocorticoid production, stress, and some bacterial and viral infections may suppress eosinophil counts (5,6). Thus, a condition promoting eosinophilia could be masked if it occurred in the presence of such events.
Eosinophils in the Tissues
Eosinophils are present in only small numbers in the circulation; they are primarily tissue-dwelling cells, with more than a hundred times as many present in the tissues as compared with the circulation (5). Eosinophils are generated in the bone marrow and reside in the hematopoietic and lymphatic organs such as the bone marrow, spleen, lymph nodes, and thymus (2). However, they are most prevalent in tissues with mucosal epithelial cells, primarily the gastrointestinal and also the respiratory and lower genitourinary tracts (4). In disease, they can accumulate in any tissue, particularly in the tissues interfacing with the environment, such as the respiratory (e.g., asthma, nasal polyps, and allergic and nonallergic rhinitis with eosinophilia), dermatologic (eosinophilic cellulitis or fasciitis), gastrointestinal (eosinophilia-associated gastrointestinal disorders), and lower genitourinary systems (eosinophilic cystitis), suggesting a role in host defense or tissue immunoregulation (7).
The peripheral blood half-life of eosinophils is as short as 8 hours to as long as 18 hours (8), but eosinophils survive for weeks within tissues (5). Thus, blood eosinophil numbers do not necessarily reflect the extent of eosinophil involvement in affected tissues in various diseases. Prolonged eosinophilia, such as in the hypereosinophilic syndrome (HES), has been associated with organ damage, particularly cardiovascular, but also cutaneous, neurologic, pulmonary, splenic, hepatic, ocular, and gastrointestinal (9).
Routine eosin staining of eosinophils may underestimate eosinophil numbers. Immunofluorescent stains with monoclonal antibodies directed against the cationic proteins from the granules or fluorescent staining techniques based on granule autofluorescense are used to detect eosinophils in tissues. Degranulation, cytolysis, apoptosis, and necrosis alter the morphology of the eosinophil and its granules and, thus, staining properties (5).
Morphology and Development of Eosinophils
To better understand the diagnosis and management of conditions characterized by eosinophilia, we present a brief synopsis of the morphology, development, and recruitment into tissue of the eosinophil. Fig. 5.1
illustrates some of these concepts. Eosinophils are bone marrow-derived granulocyte leukocytes arising from CD34+ hematopoietic progenitor cells (10). They are distinguished by their bilobed nuclei and large acidophilic cytoplasmic granules (15). The cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 are associated with promoting their growth and differentiation in the bone marrow (10). Of these, the actions of IL-5 are most specific for eosinophils, potently and specifically stimulating eosinophil production in the marrow. IL-5 stimulates eosinophil precursors to synthesize granule proteins (5), and mediates eosinophil expansion, priming, recruitment, and tissue survival (10). Despite the importance of IL-5 in eosinophilia, it is not required for eosinophil growth and differentiation under homeostatic conditions, and IL-5 gene-depleted mice produce mature eosinophils, although not in large numbers (11, 12).
illustrates some of these concepts. Eosinophils are bone marrow-derived granulocyte leukocytes arising from CD34+ hematopoietic progenitor cells (10). They are distinguished by their bilobed nuclei and large acidophilic cytoplasmic granules (15). The cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 are associated with promoting their growth and differentiation in the bone marrow (10). Of these, the actions of IL-5 are most specific for eosinophils, potently and specifically stimulating eosinophil production in the marrow. IL-5 stimulates eosinophil precursors to synthesize granule proteins (5), and mediates eosinophil expansion, priming, recruitment, and tissue survival (10). Despite the importance of IL-5 in eosinophilia, it is not required for eosinophil growth and differentiation under homeostatic conditions, and IL-5 gene-depleted mice produce mature eosinophils, although not in large numbers (11, 12).
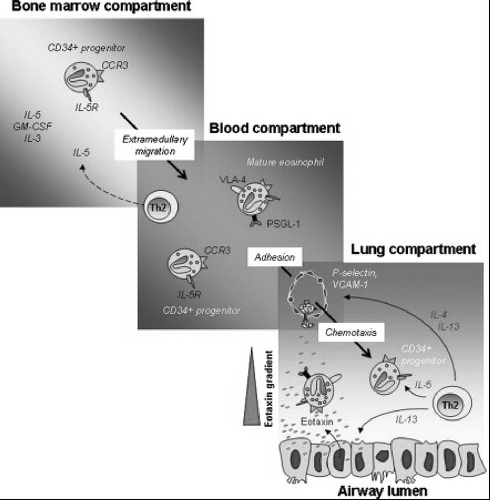 Figure 5.1 Pathways of eosinophil trafficking. Committed eosinophil progenitors (CD34+IL-5R+) differentiate and mature in response to cytokines IL-3, IL-5, and GM-CSF. Both forms exit the bone marrow and enter the bloodstream, where they respond to chemotactic signals from allergen-challenged respiratory epithelial cells. Eosinophils exit the bloodstream in response to interactions mediated by cell surface selectins and integrins and chemokine signaling and enter the lung tissue and airways. The cytokine IL-13 results in augmented expression of P-selectin and integrin VLA-4 eosinophils as well as increased P-selectin/VLA-4 dependent adherence of eosinophils to IL-4/IL-13–treated endothelial cells. Both phenotypically mature and immature eosinophil precursors can enter the lung, where the latter can differentiate into mature effector cells. Eosinophil viability is maintained by cytokines and chemokines present in the allergic lung. Reproduced with permission from: Rosenberg, H.F., S. Phipps, and P.S. Foster, Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol, 2007. 119(6): p. 1303–10. |
IL-5 and eotaxins promote the migration of eosinophils and progenitor cells through the bone marrow sinus endothelium and their release into the circulation (13). Eotaxins are chemoattractant cytokines (chemokines) that promote eosinophil recruitment to tissues via chemoattractant cytokine receptor 3 (CCR3), which is expressed predominantly on eosinophils (10). Eosinophils exit the circulation and migrate to mucosal surfaces, including the lung, gut, and lower genitourinary tract (5,10). This migration is mediated by adhesion molecules on endothelial and eosinophil surfaces. Through binding of P-selectin glycoprotein ligand 1 (PSGL-1) on eosinophils with P-selectin on endothelial cells, tethering, rolling, and margination of eosinophils occurs. The eosinophil then adheres to the endothelial wall by the binding of the β1 integrin very late antigen-4 (VLA-4) on eosinophils to the vascular cell adhesion molecule-1 (VCAM-1) (5,10). The β2 integrin, lymphocyte function-associated antigen-1 (LFA-1), which binds to intercellular adhesion molecule-1 (ICAM-1), is also important in eosinophil migration (12). With the binding of integrins and their ligands, the rolling stops, the eosinophil adheres more firmly to the endothelium and then migrates out of the vascular compartment. The migration of eosinophils into the tissues is controlled by chemoattractants. These include platelet-activating factor (PAF), complement components (C3a and C5a), leukotrienes, lipoxygenase-derived products, and chemokines. The most important group of chemokines is the eotaxins and their chemoattractive effect is augmented by IL-5. Other chemokines have also been classified as eosinophil chemoattractants. These include RANTES (chemoattractant cytokine ligand 5 [CCL5]) and macrophage inflammatory protein 1α (CCL3), but their chemoattractive properties are not specific for eosinophils. Once eosinophils migrate into inflamed tissue, they are activated by many stimuli, including receptors for immunoglobulin A (IgA) and IgG, and cytokines, including IL-3, IL-5, and GM-CSF. IL-3, IL-5, GM-CSF, and eotaxin prolong survival of eosinophils and inhibit eosinophil apoptosis (5,10,12, 13).
In tissue, eosinophils modulate immune responses using a variety of mechanisms, including antigen presentation, cytokine release, and secretion of cytotoxic granule cationic proteins (14). After engagement of their receptors by cytokines, immunoglobulins, and complement, eosinophils can release an assortment of cytokines (IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-18, TGF-α, and TGF-β), chemokines (RANTES and eotaxin-1), and lipid mediators (PAF and leukotriene C4) (14). These factors can then cause inflammation through upregulating adhesion, altering cellular trafficking, and regulating vascular permeability, mucus secretion, and smooth muscle contraction (14,15). Although eosinophils originally were considered to be effector cells participating in host defense against parasites, it is now recognized that eosinophils may also play a role in both innate and acquired immunity. They may act as initial responders to cell death and tissue damage, participate in remodeling and repair processes, and play these roles in immune responses that do not involve parasite or IgE-mediated responses (7).
Mature eosinophils can produce their end effector toxic and inflammatory effects by the release of mediators stored in their specific granules. The crystalloid core of these granules is composed of cationic major basic protein (MBP); the matrix contains eosinophil cationic protein, eosinophil-derived neurotoxin, and eosinophil peroxidase. These proteins produce hydrogen peroxide. In addition, eosinophil peroxidase generates halide acids (15). Eosinophil cationic protein can disrupt membranes by causing pore formation that facilitates the entry of other toxic molecules. It also can suppress T-cell proliferation and immunoglobulin synthesis, induce mast cell degranulation, and stimulate airway mucus secretion by fibroblasts (15). In the respiratory epithelium, activated eosinophil granule products can impair cilia beating and increase vascular permeability. MBP increases smooth muscle reactivity by acting on the epithelium and by antagonizing M2 muscarinic receptor function (16). Eosinophil granule proteins trigger degranulation of mast cells and basophils and amplify the inflammatory cascade by promoting release of chemoattractants such as eotaxin, RANTES, and PAF.
Differential Diagnosis of Eosinophilia
Eosinophilia can result from either cytokine-mediated increased differentiation and survival of eosinophils or mutation-mediated clonal expansion of eosinophils. The most common cause of eosinophilia results from an increased generation of IL-5 producing T cells regardless of the initial trigger. Eosinophilia has been
classified into primary and secondary causes (3). Primary causes include clonal eosinophilia resulting from a hematologic malignancy or idiopathic eosinophilia, which is a diagnosis of exclusion. Secondary eosinophilia includes atopic, infectious, drug-induced, vasculitic, tissue-associated inflammatory, and malignant conditions in which the eosinophils are not part of the neoplastic condition (3). Table 5.1 displays the differential diagnosis of eosinophilia in blood and tissues. It is beyond the scope of this chapter to discuss all causes of eosinophilia in detail, but Table 5.1 contains references for each. These references include the original description of disease, a review of the clinical presentation, or an update on the possible immunopathogenic mechanisms involved. Below is a review of some of the causes of eosinophilic infiltration of blood and tissues most pertinent to the allergist-immunologist and not covered in other chapters. Topics include helminthic infections, drugs, HES, Churg-Strauss syndrome (CSS), and tissue-specific eosinophilic conditions of the lung, gut, and lower genitourinary tract.
classified into primary and secondary causes (3). Primary causes include clonal eosinophilia resulting from a hematologic malignancy or idiopathic eosinophilia, which is a diagnosis of exclusion. Secondary eosinophilia includes atopic, infectious, drug-induced, vasculitic, tissue-associated inflammatory, and malignant conditions in which the eosinophils are not part of the neoplastic condition (3). Table 5.1 displays the differential diagnosis of eosinophilia in blood and tissues. It is beyond the scope of this chapter to discuss all causes of eosinophilia in detail, but Table 5.1 contains references for each. These references include the original description of disease, a review of the clinical presentation, or an update on the possible immunopathogenic mechanisms involved. Below is a review of some of the causes of eosinophilic infiltration of blood and tissues most pertinent to the allergist-immunologist and not covered in other chapters. Topics include helminthic infections, drugs, HES, Churg-Strauss syndrome (CSS), and tissue-specific eosinophilic conditions of the lung, gut, and lower genitourinary tract.
Table 5.1 Diseases Most Frequently Associated with Eosinophilia of Blood or Tissues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Infections and Eosinophilia: Helminthic Diseases
In developing countries, helminthic diseases are the most common cause of eosinophilia, whereas in developed countries, atopic diseases are most common. Infections with bacteria and most viruses are generally associated with eosinopenia. However, respiratory syncytial virus has been shown to stimulate endothelial cells to produce eosinophil chemoattractants and activate eosinophils (17). These findings may explain in part how viral infections trigger asthma exacerbations.
Parasitic, particularly helminthic, infections are associated with eosinophilia. Helminths generate TH2 responses leading to IL-4 and IL-5 production (18,19). Although IL-5 may be sufficient to cause helminth-mediated eosinophilia in most cases, helminthic infection also results in endothelial expression of eotaxin and RANTES, which promote eosinophil recruitment to tissues (19). However, the precise function of eosinophils in parasitic function in vivo is not certain. Although eosinophils have been shown to be potent effectors in killing parasites in vitro and to aggregate and degranulate in the vicinity of damaged parasites in vivo (15), in vivo data in animal models do not strongly support a requisite role for eosinophils in helminth immunity (19).
Helminthic diseases causing significant eosinophilia include strongyloidiasis, ascariasis, hookworm infection (ankylostomiasis), schistosomiasis, trichinosis, filariasis (caused by Wucheria bancrofti or Brugia species), gnathostomiasis, Toxocara canis infection causing visceral larva migrans, cysticercosis, and echinococcosis. Other helminths associated with eosinophilia include Mesocestoides corti, Hymenolepis diminuta, Angiostrongylus species, Anisakis species, Baylisascaris species, Enterobius vermicularis, Heligmosomoides polygyrus, Litomosoides species, Nippostrongylus species, Onchocerca species, Trichuris species, and Fasciola species (2,20). With the exception of Isospora belli and Dientamoeba fragilis, protozoan infections generally have not been recognized to elicit eosinophilia. Giardia does not cause eosinophilia. Eosinophilia has also been seen in Plasmodium falciparum infection (20), and recently infection with Babesia species has been suggested as a cause of eosinophilia (21). In parasitic infections associated with eosinophilia, the level of peripheral blood eosinophilia may be modest or even nonexistent if the infection is well contained in tissues such as in an echinococcal cyst. The levels of peripheral eosinophilia may fluctuate as these cysts leak or adult filaria migrate. Blood eosinophil levels tend to parallel the extent of tissue involvement and may be very marked as, for example, in disseminated Strongyloides species infection.
It is particularly important to diagnose Strongyloides species infection, which sometimes may be dormant and unrecognized in a patient for years. Potentially fatal dissemination of this helminth can occur if the patient becomes immunosuppressed or receives corticosteroids, which is the treatment for many other eosinophilic conditions (22).
Serial stool examinations with appropriate serologic tests are the initial diagnostic tests for parasites that infect the gastrointestinal tract. However, this test is not sensitive for strongyloidiasis as only small numbers of larvae are shed in the stool (20). The CDC offers a blood test with a sensitivity and negative predictive value of greater than 95% (20,23).
Eosinophilia may also be seen in primary and disseminated coccidioidomycosis (5). While eosinophilia may also be seen in HIV infected patients, it is often due to secondary causes, such as adrenal insufficiency rather than direct induction by HIV. It is also present in human T-lymphotropic virus-1 (HTLV-1) and HTLV-II infected patients (5).
Drug Reactions Associated with Eosinophilia
Table 5.2 displays drugs most commonly associated with eosinophilia of blood and tissues as an adverse reaction. Among the drugs most frequently reported are nitrofurantoin, minocycline, and nonsteroidal anti-inflammatory agents (NSAIDs). Although numerous drugs have been cited, in many cases these citations are based on case reports, as is evident from those provided in Table 5.2, making the associations difficult to interpret. When information is based on case reports, it is not clear how often eosinophilia occurs in all of the patients who take the drug. Furthermore, it is possible that a case report can describe a true association between the drug and eosinophilia, or temporally associated but causally unrelated events, such as an
occurrence of eosinophilia and a concurrent but unrelated exposure. For example, inhaled beclomethasone and cromolyn, prescribed for asthma, a disease associated with eosinophilia, have both been associated with eosinophilia in case reports (24,25). Asthma and associated eosinophilia may wax and wane as part of the disease course and this may be incidental to whether a drug is taken. In contrast, doses of systemic steroids for asthma may have been reduced when inhaled steroids were added, resulting in an increase in peripheral blood eosinophilia. Thus, the true association may be between the extent of eosinophilia and the underlying asthma, and not the drug used for treatment. Indeed, the suggested association between leukotriene antagonists and eosinophilia in asthma patients appears to result from unmasking of underlying CSS rather than a direct association between leukotriene antagonists and eosinophilia (26).
occurrence of eosinophilia and a concurrent but unrelated exposure. For example, inhaled beclomethasone and cromolyn, prescribed for asthma, a disease associated with eosinophilia, have both been associated with eosinophilia in case reports (24,25). Asthma and associated eosinophilia may wax and wane as part of the disease course and this may be incidental to whether a drug is taken. In contrast, doses of systemic steroids for asthma may have been reduced when inhaled steroids were added, resulting in an increase in peripheral blood eosinophilia. Thus, the true association may be between the extent of eosinophilia and the underlying asthma, and not the drug used for treatment. Indeed, the suggested association between leukotriene antagonists and eosinophilia in asthma patients appears to result from unmasking of underlying CSS rather than a direct association between leukotriene antagonists and eosinophilia (26).
Table 5.2 Drug-Induced Reactions Most Commonly Associated with Eosinophilia of Blood or Tissues | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
When taking a history for possible therapeutic agents associated with eosinophilia, inquiry about the use of agents used in complementary and alternative medicine and over-the-counter preparations should be made. For example, contaminated L-tryptophan and rapeseed oil were associated with the eosinophilia-myalgia syndrome (27,28). Patients should also be asked about the use of illicit drugs because cocaine and heroin use have been associated with eosinophilia. The mechanisms by which a drug might cause eosinophilia have not been fully elucidated; however, presumably it results from an increase in eosinophil growth factors such as IL-5. Investigators have associated in situ drug-induced eosinophilia with IL-5 production by infiltrating CD4 T-cells. Drug-induced eosinophilia usually resolves with discontinuation of the offending agent (1,3,28).
Hypereosinophilic Syndromes
Recently, diseases characterized by significant peripheral blood or tissue eosinophilia without identifiable precipitants were classified as hypereosinophilic syndromes by the Hypereosinophilic Syndromes Working Group (50). Included were the syndromes characterized by hypereosinophilia: HES; platelet-derived growth factor receptor α (PDGFRA)-associated HES; lymphocyte variant HES; familial hypereosinophilia; as well as CSS and EGID. The Working Group sponsored by the National Institutes of Health, a pharmaceutical company, and a nonprofit advocacy organization for those living with eosinophilic gastrointestinal disorders, aimed to promote research to identify markers of disease progression and new therapeutics for these diseases (29).
Hypereosinophilic Syndrome
HES was described in 1968 by Hardy and Anderson (30). It was a diagnosis of exclusion, characterized by eosinophilia and damage to heart, lungs, skin, or other organs infiltrated with eosinophils (31). In 1975, Chusid and colleagues (32) proposed diagnostic criteria on which the diagnosis is based today: (a) blood eosinophilia greater than 1,500/μL persisting for at least 6 months; (b) exclusion of diseases associated with eosinophilia, such as parasitic infections, HIV, allergic diseases, drug hypersensitivity, or malignancy; and (c) evidence of organ involvement. In addition, diseases generally restricted to one organ system (e.g., EGID, eosinophilic pneumonias, and eosinophilic cystitis) were excluded in this first description of HES. Although eosinophilia should be documented on more than one occasion, a delay in treatment for the 6 months required in Chusid’s criteria could be detrimental to a patient’s health (33) and a patient can be considered to have HES if he or she appears to have a chronic and unremitting clinical course (9).
HES is more common in men, with a male-to-female ratio of 9:1 (5). It usually presents between the ages of 20 and 50 years and is rare in childhood. Presenting
symptoms are often insidious and may be respiratory, cardiac, neurologic, or constitutional (such as low-grade fever or fatigue). Patients may also present with myalgias, angioedema, or skin rashes. Eosinophilia may be detected coincidentally during routine blood testing (9,34). Sweating, pruritus, abdominal discomfort, flushing, or alcohol intolerance may be seen. Weight loss is unusual, and patients do not have increased infections or anergy.
symptoms are often insidious and may be respiratory, cardiac, neurologic, or constitutional (such as low-grade fever or fatigue). Patients may also present with myalgias, angioedema, or skin rashes. Eosinophilia may be detected coincidentally during routine blood testing (9,34). Sweating, pruritus, abdominal discomfort, flushing, or alcohol intolerance may be seen. Weight loss is unusual, and patients do not have increased infections or anergy.
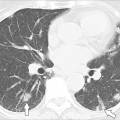
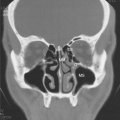
In HES, total leukocyte counts are usually less than 25,000/μL, with 30% to 70% of the total leukocyte count being eosinophils; some patients may develop very high white blood cell counts (>90,000/μL), which is associated with a poor prognosis (31). Examination of the bone marrow reveals increased numbers of eosinophils, often 30% to 60% of marrow cells. There are increased numbers of early forms compared with normal bone marrow, but blast forms are not usually present (31). When blast forms are present in the blood or make up more than 10% of the eosinophils in the marrow, the diagnosis is eosinophilic leukemia. In addition to eosinophilia, neutrophilia is common in HES. Anemia and basophilia have been described. The clinical spectrum of hematologic findings ranges from very mild abnormalities to signs and symptoms typical of a myeloproliferative disease, such as abnormal leukocyte alkaline phosphatase levels, anemia, splenomegaly (in 43% of HES patients), cytogenetic abnormalities, and myelodysplasia. Cardiac manifestations are common in HES and are present in about 58% of patients (31) (Table 5.3). Cardiac damage is thought to progress through three stages: acute necrosis, the development of endocardial thrombi, and endocardial fibrosis (31,34). The first acute stage is frequently clinically silent, although on histologic examination, damage to the endocardium including necrosis and eosinophilic infiltration of myocardium with eosinophil degranulation products and microabscesses are present. It is hypothesized that treatment in the first stage with corticosteroids will prevent progression to the other nonreversible stages (31). The second stage is characterized by thrombi in either ventricle and occasionally in the atrium. In the third stage, fibrosis may lead to entrapment of the chordae tendineae and resultant mitral or tricuspid valve insufficiency or a restrictive cardiomyopathy. Clinically, patients may present with dyspnea, chest pain, or congestive heart failure. Murmurs of mitral regurgitation can be heard. Because the heart is the most common site of organ involvement and because the first stage may be clinically silent, an electrocardiogram and echocardiogram must be obtained if HES is under consideration. Serial echocardiograms should be used to monitor cardiac involvement in patients in whom HES is a diagnostic possibility and in those with established disease. Cardiac MRI may prove to be a more reliable test for the rapid noninvasive diagnosis of myocardial complications of hypereosinophilic syndrome (35).
Table 5.3 Distribution of Clinical Manifestations in Hypereosinophilic Syndrome | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
The most common cutaneous findings are erythroderma, urticarial plaques, angioedema, pruritic papules, and nodules (31,34). A less common manifestation is mucosal ulcerations that can be difficult to treat and are found most often in the myeloproliferative variant (34). Patients who have urticaria or angioedema as skin manifestations tend to have a better prognosis; they are more likely to have no cardiac or neurologic manifestations (531,34). Biopsy specimens of the papular and nodular lesions are characterized by perivascular infiltrates of eosinophils, neutrophils, and mononuclear cells without evidence of vasculitis (31).
Neurologic involvement occurs in about half of cases and has three forms: thromboembolic disease resulting from thrombotic cardiac disease, primary central nervous system dysfunction, and peripheral neuropathy (31




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree