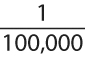Evaluation and Management of Immune Deficiency in Allergy Practice
Melvin Berger
There is considerable overlap between the manifestations of allergy and respiratory infection (i.e., rhinorrhea, sneezing, coughing, wheezing), and allergy may be a predisposing factor in sinusitis, otitis, and other respiratory infections. Therefore, the allergist must frequently evaluate patients with symptoms that have been attributed to recurrent infections and in which the competence of the patient’s immune system has been or should be questioned. Because half or more of all patients with primary immune defects have antibody deficiencies (1,2) and most of them have problems with recurrent sinopulmonary infections, this is not an uncommon condition for which allergist-immunologists are consulted. Recent surveys suggest that the population prevalence of diagnosed primary immune deficiencies in the United States is at least 1 in 1,200, and many additional cases are undiagnosed (3). The intent of this chapter is to provide a practical approach to the diagnosis and management of such patients, not a comprehensive review of immune deficiency disorders or their molecular bases. Readers who wish a more in-depth analysis of immune deficiency disorders should consult comprehensive texts such as those edited by Ochs et al. (4) or Stiehm et al. (5).
Indications for an Immunologic Workup
Although many immune-deficient patients present with a clear history of distinct episodes of severe infection, the allergist is frequently called on to see patients with less severe, nonspecific symptoms such as nasal stuffiness, chronic and recurrent rhinorrhea, or cough, which may be due to infection, allergy, or other factors. The first step in sorting out such complaints is to try to distinguish whether the symptoms are, in fact, due to infection. Inciting factors, such as seasonality, and clearly identifiable triggers may suggest allergic etiologies; but changes in the weather and changes in seasons are frequently accompanied by changes in exposure to infectious diseases, especially among school-aged children.
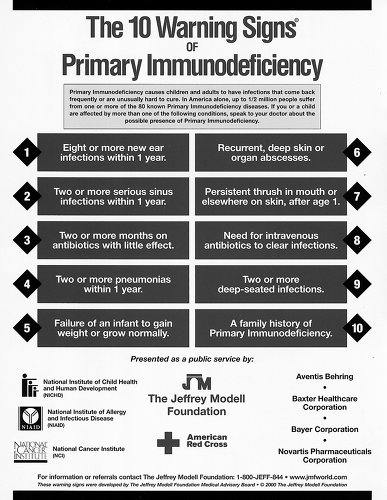
A history of exposure to other people with similar symptoms; and details such as the presence or absence of fever, description of excessive secretions (clear and watery versus thick and purulent), and the response to antibiotics, may help to distinguish between infectious and noninfectious etiologies. After an estimate of the real incidence of infection is obtained, this can be compared with benchmarks such as the “10 Warning Signs of Immune Deficiency” (Fig. 4.1). The incidence of infection should be compared with the incidence for that age group in the community, but the exposure history should also be considered. For example, a 40-year-old who lives alone and sits at a computer all day would be expected to have a different degree of exposure to infectious agents than a kindergarten teacher, day care worker, or pediatric office nurse. College students moving from home to the dormitory for the first time and military recruits often have sharp increases in infectious disease exposure. Similarly, a first-born baby at home often has a very different degree of exposure than a similar-aged child in day care or with many siblings. Generally, the frequency of respiratory infection among school-aged children in the United States is about six to eight upper respiratory infections per year, but as many as one a month while school is in session is not unusual. About half of these may be primary bacterial infections or secondary bacterial sequelae, such as otitis media, sinusitis, pneumonia, or bronchitis.
Patients with clear histories of more than 10 distinct episodes of infection per year, more than two documented episodes of pneumonia per year, or more than
one life-threatening infection should be evaluated for possible immune deficiency or other underlying abnormalities. However, the specialist must be careful in interpreting the history from the patient or parent. Frequently, antibiotics are given when the patient does not have clear evidence for bacterial infection, then a failure
to respond leads to the conclusion that “antibiotics do not work.” This may, in turn, lead to the suggestion that there is something “wrong” with the patient’s immune system. Frequent upper respiratory symptoms may represent individual viral upper respiratory infections. On the other hand, there may be prolonged symptoms from chronic infections such as sinusitis that have not been adequately treated despite multiple short courses of oral antibiotics. Patients who present with the complaint of “constant colds” may actually have allergic rhinitis. Densities on chest radiograph may represent atelectasis due to asthma rather than true infiltrates, and should not necessarily be taken as indicating recurrent pneumonia unless there is documentation of concomitant fever, elevated white blood cell count, or positive sputum Gram stain or culture.
one life-threatening infection should be evaluated for possible immune deficiency or other underlying abnormalities. However, the specialist must be careful in interpreting the history from the patient or parent. Frequently, antibiotics are given when the patient does not have clear evidence for bacterial infection, then a failure
to respond leads to the conclusion that “antibiotics do not work.” This may, in turn, lead to the suggestion that there is something “wrong” with the patient’s immune system. Frequent upper respiratory symptoms may represent individual viral upper respiratory infections. On the other hand, there may be prolonged symptoms from chronic infections such as sinusitis that have not been adequately treated despite multiple short courses of oral antibiotics. Patients who present with the complaint of “constant colds” may actually have allergic rhinitis. Densities on chest radiograph may represent atelectasis due to asthma rather than true infiltrates, and should not necessarily be taken as indicating recurrent pneumonia unless there is documentation of concomitant fever, elevated white blood cell count, or positive sputum Gram stain or culture.
Patients with unusually severe infections, such as those requiring parenteral antibiotics, prolonged or multiple courses of antibiotics for a single infection, or surgical intervention such as incision and drainage of abscesses or removal of seriously infected tissue (e.g., a segment of lung or infected bone), should probably also undergo at least screening (see later) to exclude immune deficiency. Patients with unusual or opportunistic infections, or with unusual responses, such as prostration or excessive fever to seemingly common organisms, should also be evaluated for immune deficiency.
Although many patients with primary immune deficiencies present with recurrent and chronic respiratory infections (2,5–7), gastrointestinal disorders are also common in these patients (8,9). The combination of recurrent respiratory infections with recurrent gastrointestinal symptoms may prompt immunologic screening even when the involvement of either organ system itself is not severe. Infection with Giardia lamblia (7,8) and bacterial overgrowth in the small intestine are not infrequent in patients with antibody deficiencies. These problems may present with symptoms such as cramps or diarrhea after eating, leading to suspicion of food allergy despite the absence of other manifestations of IgE-mediated reactions. In some immune-deficient patients, there may be organized lymphonodular hyperplasia in the intestine or infiltration of the submucosa with scattered aggregates of lymphocytes (7). Patients with gastrointestinal workups or biopsy results not typical for recognized patterns of inflammatory bowel disease should also undergo evaluation for immune deficiencies.
TABLE 4.1 Physical Findings Not Due to Infectious Disease Associated with Selected Immune Deficiency Syndromes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The presence of nonimmunologic findings on physical examination may also provide indications for evaluation to exclude immune deficiency (Table 4.1). Failure to thrive and/or leveling out of the growth curve in children, and unexplained weight loss in adults may indicate malabsorption due to intestinal infection or cumulative morbidity of other infections. The importance of recording accurate measurements of weight and height in children at every visit cannot be overemphasized. Eczema and thrombocytopenia may suggest Wiskott-Aldrich syndrome (10). Facial, cardiac, or skeletal features are often suggestive of a recognizable pattern of malformation such as that seen in DiGeorge syndrome, short-limbed dwarfism, or cartilage-hair hypoplasia (11,12). Characteristic abnormalities of dentition have been described in NF-κB essential modulator (NEMO) deficiency and hyper IgE (Job) syndrome (13–15). The latter is also often accompanied by facial and skeletal abnormalities and a unique form of eczematoid dermatitis (14,15). Rib flaring and prominent costochondral junctions are skeletal abnormalities that may be present in severe combined immune deficiency (SCID) due to adenosine deaminase (ADA) deficiency (16). Alopecia and/or endocrinopathies occur with increased frequency in chronic mucocutaneous candidiasis due to mutations in the AIRE gene (17). Nystagmus, clumsiness, and other neurologic abnormalities may occur before observable telangiectasias and can suggest the diagnosis of ataxia-telangiectasia (18). Neurologic disorders are also common in purine nucleoside phosphorylase deficiency (19). Although delayed separation of the umbilical cord stump is widely recognized as an indicator of leukocyte adherence protein deficiency, in fact, there is a wide variation in the time at which the stump separates, and this should not be overemphasized in an otherwise well infant (20). Of course, patients with positive screening tests for human immunodeficiency virus (HIV) would also be candidates for immunologic evaluation.
Several immune deficiencies are clearly hereditary. For many, the patterns of inheritance and the precise molecular defects have been defined (21,22) (Table 4.2). Family members suspected of having these disorders, perhaps because an older sibling has already been diagnosed, should undergo assessment of their immune status. When available, tests for the specific molecular lesion should be included so that treatment aimed at correcting or compensating for the basic defect can be instituted early enough to prevent or minimize end-organ damage. Prenatal diagnosis and screening for the carrier state is now available for many of these disorders and can be used both in counseling and in ensuring that prompt and appropriate therapy is offered to affected newborns. It is important to realize, however, that a negative family history does not rule out a disease that is usually considered hereditary. For example, analysis of a large group of patients with confirmed mutations in Bruton tyrosine kinase revealed that 73% of the cases were sporadic, indicating a new mutation on the x-chromosome in that particular patient (6).
TABLE 4.2 Major Inherited Immune Deficiencies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Documenting the History of Infection
A major goal in questioning the patient and reviewing the medical records is to develop a firm impression of
the types of infections that the patient has suffered, so that subsequent laboratory tests can be targeted to specifically analyze those components of the immune system whose defects would most likely explain the patient’s symptoms. This will be best served by keeping in mind general patterns of infection that might be caused by defects in specific immunologic defense mechanisms. Thus, infections with encapsulated extracellular bacterial pathogens, particularly of the respiratory tract, are suggestive of defects in antibody production (23), which constitute the majority of all immune deficiencies (1). Noninvasive mucosal infections may particularly suggest isolated IgA deficiency (24). Infections with opportunistic pathogens, including protozoans and fungi, and severe or recurrent episodes of chickenpox or herpetic lesions, may suggest problems in cell-mediated immunity (4–7). Failure to clear bacteria promptly from the blood stream, resulting in bacteremia/sepsis, or hematogenously disseminated infections such as osteomyelitis, may be seen in deficiencies of C3 or early-acting
components of the complement system (25), but may also indicate asplenia or poor reticuloendothelial system function, as in sickle cell disease. Recurrent or disseminated neisserial infections may suggest deficiency of the later-acting complement components that form the membrane attack complex (25). Abscesses and infections with unusual bacteria or fungi may suggest neutropenia or defects in neutrophil function (6,7,26–28). Enteroviral meningoencephalitis may suggest X-linked agammaglobulinemia (29). On the other hand, it should be remembered that normal babies have increased susceptibility to infections usually controlled
by T cells and γ-interferon (30), so that isolation of some organisms otherwise considered “opportunistic” should not always be cause for alarm.
the types of infections that the patient has suffered, so that subsequent laboratory tests can be targeted to specifically analyze those components of the immune system whose defects would most likely explain the patient’s symptoms. This will be best served by keeping in mind general patterns of infection that might be caused by defects in specific immunologic defense mechanisms. Thus, infections with encapsulated extracellular bacterial pathogens, particularly of the respiratory tract, are suggestive of defects in antibody production (23), which constitute the majority of all immune deficiencies (1). Noninvasive mucosal infections may particularly suggest isolated IgA deficiency (24). Infections with opportunistic pathogens, including protozoans and fungi, and severe or recurrent episodes of chickenpox or herpetic lesions, may suggest problems in cell-mediated immunity (4–7). Failure to clear bacteria promptly from the blood stream, resulting in bacteremia/sepsis, or hematogenously disseminated infections such as osteomyelitis, may be seen in deficiencies of C3 or early-acting
components of the complement system (25), but may also indicate asplenia or poor reticuloendothelial system function, as in sickle cell disease. Recurrent or disseminated neisserial infections may suggest deficiency of the later-acting complement components that form the membrane attack complex (25). Abscesses and infections with unusual bacteria or fungi may suggest neutropenia or defects in neutrophil function (6,7,26–28). Enteroviral meningoencephalitis may suggest X-linked agammaglobulinemia (29). On the other hand, it should be remembered that normal babies have increased susceptibility to infections usually controlled
by T cells and γ-interferon (30), so that isolation of some organisms otherwise considered “opportunistic” should not always be cause for alarm.
The number and types of infections and their individual and cumulative morbidity should be assessed. It is necessary to carefully exclude other causes of nonspecific symptoms; for example, is sniffling or congestion due to recurrent upper respiratory infection, allergy, or other types of rhinitis? In contrast, documentable sinusitis is a frequent complication of primary immune deficiency (31). If cough is a major complaint, it is important to determine whether this is due to sputum production versus irritation, or other causes. Could it represent cough-equivalent asthma? If failure to thrive and cough are both present, could the patient have cystic fibrosis? Celiac or other forms of inflammatory bowel disease may mimic hypogammaglobulinemia in children with poor weight gain who also have frequent upper respiratory infections, which by themselves would not be considered significant.
Isolation and identification of responsible organisms is clearly the gold standard for rigorous diagnosis of infection. Documentation of fever, white blood count with differential, and sensitive but nonspecific measures such as the erythrocyte sedimentation rate and C-reactive protein can help distinguish between recurrent/chronic sinusitis and headaches due to other causes. These tests can also help with the differential diagnosis of recurrent cough or other chest symptoms. The importance of culture and examination of smears of nasal secretions for bacteria and neutrophils versus eosinophils cannot be overemphasized in distinguishing infectious from allergic and other noninfectious etiologies, particularly in small children. In some cases, the most appropriate step in the workup is to send the patient back to the primary care physician with instructions to have appropriate cultures and the readily available laboratory tests listed above performed every time an infection is suspected or the symptoms recur. Similar steps may also help in identifying adults with recurrent headaches erroneously attributed to chronic/recurrent sinusitis. Sometimes, culture results point to the diagnosis, as in the case of Pseudomonas aeruginosa suggesting cystic fibrosis, or invasive aspergillosis suggesting neutropenia or chronic granulomatous disease (CGD) (28). Chronic or recurrent Cryptosporidium parvum infection may suggest the X-linked hyper IgM syndrome (CD40 ligand deficiency) (32,33), and, of course, Streptococcus pneumoniae or Haemophilus influenzae suggest antibody deficiency (1,5–7,23).
Clues to the severity and overall morbidity resulting from infection may be obtained by asking whether hospitalization or intravenous antibiotics have been required to treat infections or whether oral antibiotics have generally been sufficient. The response to therapy should be evaluated carefully. Continued high fever or other symptoms suggesting a lack of response of culture-confirmed bacterial infection to antibiotics is more likely indicative of a significant immune deficiency than is the frequently seen pattern in which the fever and symptoms resolve promptly when antibiotic therapy is started (e.g., for otitis media) only to recur again shortly after the prescribed course of therapy is concluded. The latter may actually represent a distinct new infection. This pattern is quite commonly seen in children in day care and in adults with frequent exposure to small children. Similarly, it is also important to distinguish inadequate or inappropriate therapy (i.e., antibiotics for viral URIs) from failure to respond, and it is important to differentiate chronic infections from recurrent episodes. Absence from school or work should be quantitated if possible, and any long-term sequelae or disability should be documented. The family history should include questions about siblings and preceding generations. Family trees with premature deaths of male infants should raise suspicion of X-linked immune deficiencies (Table 4.2). However, the absence of such a family history does not rule out X-linked disorders, which may have a high spontaneous mutation rate (6,22). Questions should also be asked about the family history of asthma and allergy as well as other genetic diseases that may present with recurrent infection such as cystic fibrosis. In evaluating a child, it may be important to determine whether the parents have died prematurely or have known risk factors for HIV infection.
The age at onset of infections of unusual frequency or severity may yield important insights into possible underlying immune deficiencies. It must be kept in mind that term newborns have IgG levels equivalent to those of their mothers, from whom most of their IgG has been transferred across the placenta (34). Thus, babies who have problems with infections during the first few months of life may have T-cell or phagocyte problems but are less likely to have agammaglobulinemia or other isolated problems in antibody production. In contrast, disorders of antibody production are more likely to present after the age of 6 months. The history of exposure must be carefully considered in evaluating this issue because the frequency of common types of infections often increases after a child’s exposure to infectious agents increases on starting day care or preschool, particularly if there are no siblings in the home. Although patients with severe antibody deficiency such as that seen in Bruton agammaglobulinemia classically present between 6 months and 2 years of age (6,29), that diagnosis as well as the diagnosis of hyper-IgM syndromes is often delayed until later in childhood (6,29,31,32). Common variable immunodeficiency disease (CVID) may present at any age (35–37). That diagnosis is frequently delayed by 8 to 10 years from the onset of increased morbidity due to infection. Diagnosis of CVID in older children and young adults may represent an early-onset deficiency that has not been
previously recognized or a newly acquired problem. Just as some infants may have delayed development of the full range of immune responses (38), it seems likely that some adults may undergo premature senescence of immune responsiveness (39) and may present with recurrent bacterial infections and/or activation of latent infections (i.e., shingles, tuberculosis) in their 40s or 50s, as opposed to their 60s or 70s.
previously recognized or a newly acquired problem. Just as some infants may have delayed development of the full range of immune responses (38), it seems likely that some adults may undergo premature senescence of immune responsiveness (39) and may present with recurrent bacterial infections and/or activation of latent infections (i.e., shingles, tuberculosis) in their 40s or 50s, as opposed to their 60s or 70s.
The Physical Examination in Cases of Suspected Immune Deficiency
The physical examination often provides important evidence for or against immune deficiency and may also allow the physician to assess critically the cumulative morbidity due to infection. Most importantly, the presence or absence of lymphoid tissue should be carefully documented. The absence of visible tonsils in patients who have not had them surgically removed and the absence of palpable cervical or inguinal lymph nodes should promote a strong suspicion of a significant antibody deficiency because the bulk of these tissues is composed of B-lymphocytes involved in antibody synthesis. Conversely, the presence of palpable lymph nodes and easily visible tonsils essentially excludes Bruton agammaglobulinemia and may suggest the absence of SCID, but does not help one way or the other with the diagnosis of CVID or X-linked hyper-IgM syndrome. The presence of cervical or peripheral adenopathy, splenomegaly, or hepatomegaly may suggest CVID, HIV, CGD, or other abnormalities. Many anatomic findings are associated with immune defects in recognizable malformation syndromes (Table 4.1); characteristic rashes may suggest Wiskott-Aldrich (10) or hyper-IgE (Job) syndromes (14,15); clumsiness, nystagmus and ocular telangiectases may suggest ataxia-telangiectasia (18); and craniofacial abnormailities may suggest DiGeorge syndrome in patients who do not have major cardiac defects (12). Secondary effects, such as failure to thrive, weight loss, and short stature, may suggest significant morbidity due to chronic or recurrent infection. Scars from incision and drainage of abscesses or from drainage or surgical reduction of enlarged lymph nodes may indicate significant morbidity from neutrophil defects (27,28).
Autoimmune phenomena (40,41) and rheumatic complaints (40–42), including infectious or chronic arthritis, are common in patients with CVID and other primary immune deficiencies and may suggest evaluation for immune deficiency, even if the number or severity of acute infections has not been excessive.
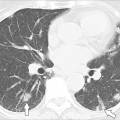
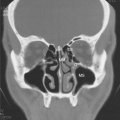
Careful assessment of the tympanic membranes, paranasal sinuses, and chest is extremely important in evaluating patients suspected of having antibody deficiency syndromes. The quantity and characteristics of secretions should be documented and it should determined whether observed abnormalities are acute or chronic. In this regard, high-resolution (thin-slice) computed tomography (CT) scans of the chest and formal pulmonary function testing may be very helpful, because observation of bronchiectasis, areas of “ground-glass” density in the lung parenchyma, and/or hilar adenopathy may suggest the presence of subclinical chronic disease, which could be associated with antibody deficiency and/or CVID (43,44). Clubbing of the digits may also provide an important indication of chronic lung disease.
General Laboratory Screening Tests
Guidelines for the diagnosis and management of immunodeficiency and handy algorithims are available from the Joint Council on Allergy, Asthma, and Immunology (45), the Immune Deficiency Foundation (1), and the Jeffrey Modell Foundation (http://www.info4PI.org). These can help prioritize screening tests that might be ordered and interpreted by the primary physician and define situations in which referral to the specialist becomes appropriate. Often, the primary care physician can already have these results in hand when the specialist is called to determine if referral is appropriate.
A review of laboratory tests already obtained by the primary care physician may yield important clues to the presence of an immune deficiency disorder and may save steps in the evaluation of patients by suggesting which of the more specialized tests are most likely to be informative. The complete blood count (CBC) and differential is a critical first step. It is important to remember that lymphocyte counts in newborns should be higher than in older children and adults, and that age-appropriate norms should be used (46




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree