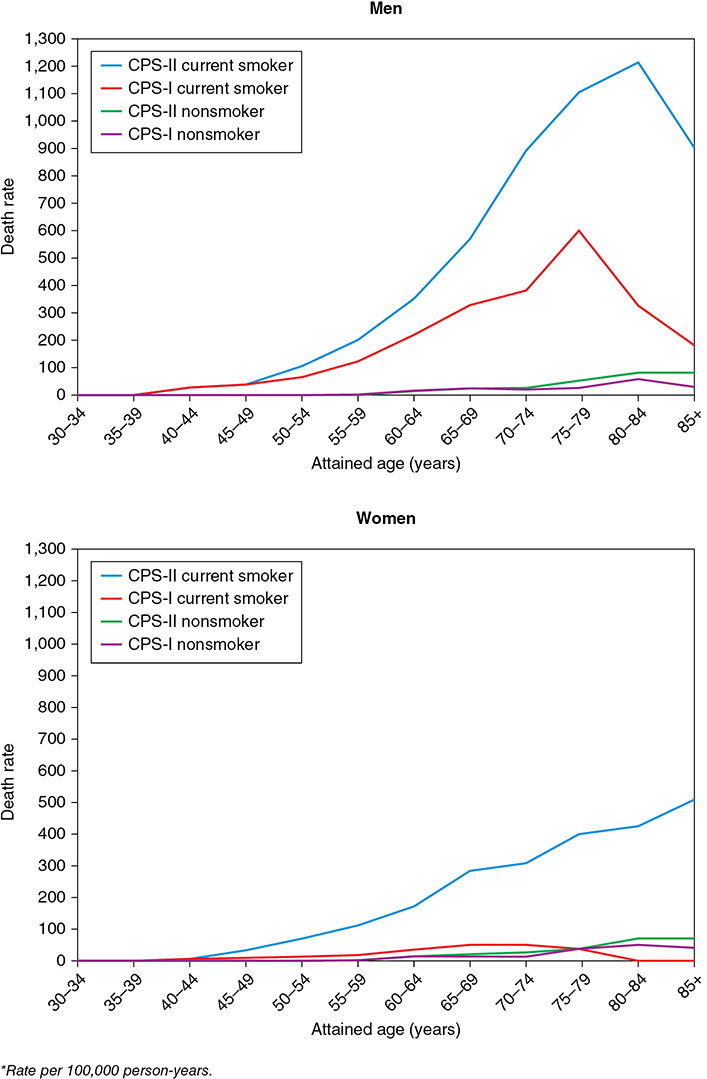
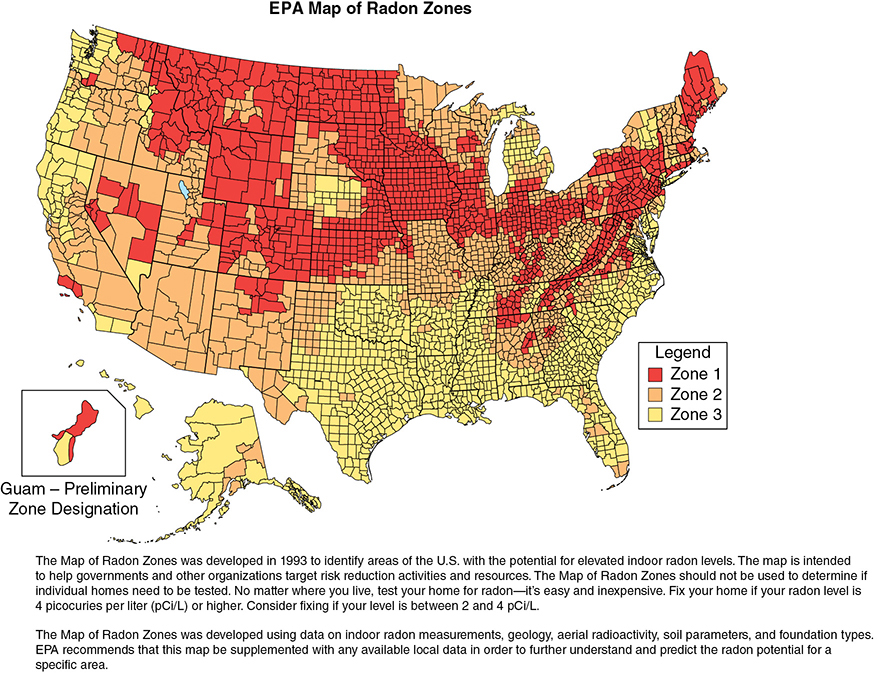
Your 60-year-old female patient with metastatic non-small cell lung cancer never smoked. She has been healthy all her life, and her social, family, and professional history is noncontributory. She asks you why she has lung cancer. Learning Objectives: 1. What are the risk factors for lung cancer? 2. What is the second most common cause of lung cancer? 3. How frequently does asbestos cause lung cancer? 4. What professional exposures are risk factors? Tobacco smoking accounts for 80%-90% of all lung cancer incidence, with a reported 20- to 30-fold increased risk in smokers compared to non-smokers. This is by far the most thoroughly established causal relationship in medical literature. There is a lag period of approximately 20 years between the exposure and disease occurrence.1 The widespread use of tobacco in the form of cigarette smoking started after the invention of the cigarette-rolling machine in the late 19th century. In the mid-20th century, 2 large epidemiological studies established a definite etiologic role of tobacco smoking in lung cancer.2,3 This led to the US surgeon general issuing a public safety warning in 1964 regarding the potential harmful effects of tobacco smoking and then reemphasizing its stance in 2004.4 A combined effort has led to a significant decline in tobacco smoking: from 42.4% of the adult population in 1965 to 14.0% of all adults in 2017 (15.8% of men, 12.2% of women smokers).5 Since the 1950s, the cigarette has significantly evolved, with a shift to its filtered use. There has also been a decrease in the tar and nicotine contents as per machine-measured yields. Unfortunately, these changes have not resulted in a decrease in lung cancer risk or mortality, as evident by the results of the Cancer Prevention Studies (CPSs). In CPS-1 follow-up period 1960-1972, lung cancer mortality risk was compared based on tar yield of the products. The mortality in the low- and medium-yield group was 20% lower than that in the high-yield group. In CPS-2 follow-up period 1980-1986, with the composition of cigarettes changing, it was expected that there would be a decrease in mortality. To the contrary, the mortality was higher in CPS-2 (Figure 3-1). Figure 3-1. Age-specific death rates from lung cancer among current cigarette smokers and lifelong never-smokers. (Reproduced with permission from Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1)(suppl):21s-49s; Adapted from Burns DM, Garfinkel L, Samet JM, eds. Changes in Cigarette Related Disease Risks and Their Implications for Prevention and Control. Bethesda, MD: US Government Printing Office; 1997: 317.) It has been suggested that the change in smoking habits or more smoking as low-yield products were thought to be “safer” may have dampened any benefits of the low-yield cigarettes. Overall, there has been no benefit of changing cigarette design and composition.1 Tobacco contains a psychoactive compound called nicotine that causes addiction but itself is not carcinogenic. It is an acetylcholine agonist that causes release of endorphins and neurotransmitters into the bloodstream, leading to dependence. It has also been associated with progression of a preexisting lung tumor.6–8 Menthol is a frequent additive to tobacco products to make cigarettes more palatable with mint flavor and reduced mucosal irritation. These products were specially targeted to females, African Americans, and young adults. Besides its soothing effects, menthol upregulates cholinergic receptors and their binding to nicotine, enhancing the addictive effects. It also promotes aggressive smoking habits. Regulatory authorities have proposed a ban on menthol additive altogether.9,10 The International Agency for Research on Cancer (IARC) has identified at least 50 carcinogens in tobacco smoke. Mainstream smoke contains many potential carcinogens, including polycyclic aromatic hydrocarbons (PAHs), aromatic amines, N-nitrosamines, and other organic and inorganic compounds (eg, benzene, vinyl chloride, arsenic, and chromium). Of particular importance are the N-nitrosamines, especially 4-(methylnitrosamino)-1(3-pyridyl)-1-butanone (NNK), which has been shown to induce adenocarcinoma in animal models.9,11 Tobacco smoking can cause all histologic subtypes of lung cancer. In the last 50 years, there has been a shift in histologic characteristics, with adenocarcinoma taking over squamous cell carcinoma to become the most common subtype. The adenocarcinoma tends to arise more peripherally and squamous cell carcinoma more centrally. This shift is thought to be due to introduction of low-tar filter cigarettes and changes in smoking topography that have increased exposure of peripheral lung tissue to the carcinogens.11 The cumulative incidence of lung cancer was 31.7% in male and 15.3% in female heavy smokers compared to 0.9% in male and 0.5% in female non-smokers.12 The duration of smoking is a much stronger risk factor than the quantity smoked. For example, a 3-fold increase in quantity of tobacco smoking is associated with a 3-fold increase in lung cancer risk, while an increase in duration of exposure by 3 times has a 100-fold increase in the risk.13 Approximately 90% of all lung cancer mortality is attributable to tobacco smoking.14 Smoking cessation significantly decreases (up to 90%) lung cancer risk but remains higher than never-smokers even after more than 50 years of abstinence.11,15 Other forms of tobacco smoking like cigars and pipes are also associated with lung cancer risk but to a lesser extent compared to cigarette smoking. This likely is due to less smoking frequency and depth of inhalation. On average, smoking 5 cigars a day is equivalent to 1 pack a day of cigarettes.11,13 Passive or secondhand smoking can also contribute to increased lung cancer risk. Various carcinogens, like benzene, benzo[a]pyrene, and 4-(methylnitrosamino)-1-(13-pyridyl-1-butanone), have been identified in environmental tobacco smoke (ETS).13 The US Environmental Protection Agency (EPA) and the IARC have identified that ETS contains lung carcinogen. In an analysis of 37 published epidemiological studies, the authors found that the excess risk of lung cancer was 24% (95% CI 13%-36%, p < .001) in lifelong non-smokers who were exposed to secondhand smoke. The dose-response relation of the risk of lung cancer with both the number of cigarettes smoked by the spouse and the duration of exposure was significant.16 It has been estimated that lung cancer deaths attributable to ETS is comparable to that of radon or asbestos.17 Electronic cigarettes (e-cigs) are battery-operated nicotine delivery systems. The system consists of a heating element and replaceable cartridges filled with liquid nicotine, propylene glycol or glycerol, and flavorings. There may be other carcinogenic metals and organic compounds. The heating coil produces vapors from the liquid that are inhaled by the user. E-cigs were introduced to the US market in 2007 and have been largely unregulated except for mandated age checks. In 2016, the US Food and Drug Administration (FDA) claimed jurisdiction and regulatory authority over the manufacture, promotion, sale, and distribution, although compliance is yet to be enforced and may not include all manufacturers.18 Although advertised as the “safer option,” potential long-term side effects are not yet known. Use of e-cigs has been shown to be more effective than traditional nicotine replacement therapy. In a recently published randomized control trial of 886 participants, the 1-year abstinence rate was higher in the e-cig group compared to 9.9% for the nicotine replacement group (18.0% vs 9.9%) and had a relative risk (RR) of 1.83 (95% CI 1.30-2.58).19 The contents of e-cig vapors are different from tobacco smoke, but prolonged exposure to organic compounds, trace metals, and reactive oxygen species can cause chronic inflammation of the respiratory epithelium. Overall, 15.3% of adults aged 18 years or older has ever used an e-cig, and 3.2% currently used e-cigs in 2016. Adults aged 18-24 years were the most likely to have ever used an e-cig (23.8%).20 There have been growing concerns regarding the rapid increase in use of e-cigs in adolescents and young adults who were previous non-smokers of traditional tobacco. Furthermore, recent studies have shown initiation of traditional tobacco smoking in previous non-smokers who start using e-cig.21 Current Centers for Disease Control and Prevention (CDC) recommendations are that e-cigs have the potential to benefit adult smokers who are not pregnant if used as a complete substitute for regular cigarettes and other smoked tobacco products. E-cigarettes are not safe for youth, young adults, pregnant women, or adults who do not currently use tobacco products.22 Marijuana is the most commonly used illicit drug in the United States.23 The landscape of marijuana use is rapidly changing with the increase in its legalization for recreational use. Cannabis contains a psychoactive substance known as tetrahydrocannabinol or THC that, like nicotine, has addictive properties but has not been directly associated with carcinogenesis. Approximately 9% of those who experiment with marijuana will become dependent.24 Similar to tobacco smoke, marijuana smoke has several potent carcinogens. Furthermore, the latter may be even more cytotoxic and mutagenic.25 The tar and PAH content of marijuana smoke is also higher than that of tobacco.9,26 The smoking technique of marijuana is significantly different from tobacco, involving deep inhalation and longer breath-holding times, and it is frequently used without filters. In addition, experimental studies employing bronchial biopsies have demonstrated that marijuana users manifest not only airway inflammation, but also histopathological or molecular changes indicative of precancerous bronchial activity.27,28 Even though there are several experimental studies that provide biological evidence of increased lung cancer risk associated with marijuana, the epidemiological studies of this association have been inconsistent. This is partly due to a small sample size, previous illegal status of marijuana, and several confounding effects of tobacco smoking, including tobacco mixing with marijuana in some regions. In a Swedish 40-year cohort study of military conscripts, there was no significant increased risk of lung cancer in “ever” versus “never” marijuana smokers after adjustment for baseline tobacco use, alcohol use, respiratory conditions, and socioeconomic status. In the subset of heavy cannabis smokers (>50 times), there was a 2-fold increase in risk of lung cancer (adjusted hazard ratio [HR] 2.12, 95% CI 1.08-4.14) over the 40-year follow-up period.29 The cohort study on lung cancer reported an increased risk for marijuana use with a dose-response evaluation for the number of times used in a lifetime, but “lifetime” use was assessed only up to the ages of 18 to 20 years, with no information on subsequent use over the 40-year follow-up period and no dose-response evaluation for frequency. In a pooled analysis of 6 case-controlled studies from the United States, Canada, United Kingdom, and New Zealand, data on 2,159 lung cancer cases and 2,985 controls analyzed by Zhang et al. The overall pooled odds ratio (OR) for habitual versus non-habitual or never users was 0.96 (95% CI, 0.66-1.38).30 There is currently no consensus on whether marijuana use is directly associated with increased lung cancer risk, but there are data supporting increased tobacco use associated with marijuana smoking in adolescents and young adults.31,32 With the industrialization of major cities throughout the world in the mid- to late 1900s, an increase in lung cancer incidence raised the question of pollution as a risk factor for developing lung cancer. Particulate matter, in regard to air pollution, is a term used to describe the mixture of small, solid particles and liquid droplets in the air that comes from sources such as power plants and automobiles. In the United States, the awareness was first raised when the Clean Air Act Amendments in 1977 required the EPA review scientific criteria for ambient air pollution to identify air quality standards. A report from the EPA analyzed size differences in different particles to determine which inhaled particle sizes were the most detrimental to human health. The report showed that there were 2 necessary cutoffs for inhalable particles, 15 and 2.5 μm. Particles greater than 15 μm were retained in the upper respiratory tract and had minimal lower respiratory tract penetration.33 Particles less than 2.5 μm were classified as fine particles and penetrated deeper into the respiratory tract. The major components of fine particles are sulfate, ammonium, and nitrate ions as well as more carcinogenic compounds, such as arsenic and selenium.34 One large study in the United States followed 8,111 adults in 6 different cities for over 14 years and found air pollution to be positively associated with increased death from lung cancer and cardiovascular death.35 In 2013, a large prospective analysis of 17 European populations called the European Study of Cohorts for Air Pollution Effects (ESCAPE) followed over 300,000 people for an average of 12.8 years and analyzed associations with exposure to different size particulate matter.36 The risk for lung cancer with particulate matter less than 10 μm was HR 1.22, 95% CI 1.03-1.45 per 10 μg/m3; for particles less than 2.5 μm, the HR was 1.18 (0.96-1.46) per 5 μg/m3.36 In an another study, a 10-μg/m3 change in fine particles measuring less than 2.5 μm has been associated with an 8% increase in lung cancer mortality after adjusting for various factors, including smoking status.37 The risk of lung cancer from pollution is further increased with tobacco smoking. With an overwhelming amount of data confirming associations between particulate matter and lung cancer, the World Health Organization (WHO) in conjunction with the IARC officially declared particulate matter as carcinogenic to humans.38 In the year 2015, ambient air pollution was attributable for 4.4 (95% CI 2.7-6.1) age-adjusted lung cancer deaths per 100,000 people worldwide.39 In many developing countries, fossil and plant-based fuels have been the primary source of domestic energy for heating and cooking. Indoor pollution due to burning of these fuels, along with poor ventilation, has been associated with lung cancer risk. In a case-control study of rural China, the OR for lung cancer associated with coal use compared with that for biomass (crop residues, wood, sticks, and twigs) in the house of longest residence was 1.29 (95% CI 1.03-1.61), adjusted for smoking and socioeconomic status. The risk for lung cancer increased relative to the percentage of time that coal was used over the past 30 years (p = .02).40 In a pooled analysis from International Lung Cancer Consortium, predominant coal users (OR = 1.64, 95% CI 1.49-1.81)—particularly coal users in Asia (OR = 4.93, 95% CI 3.73-6.52)—and predominant wood users in North American and European countries (OR = 1.21; 95% CI 1.06-1.38) experienced a higher risk of lung cancer when compared to non–solid fuel user (oil, gas, electricity).41 The IARC has classified indoor emissions from household coal combustion as human carcinogens. Asbestos is a term used to refer to a collection of naturally occurring minerals that share a fibrous nature in their composition. It is the most common occupational cause of lung cancer. It consists of 2 subtypes: serpentine (chrysotile) and amphibole (amosite, crocidolite, and tremolites). Asbestos materials are largely inert and non-flammable and have a tensile strength stronger than steel. These properties have led to it being incorporated into over 3,000 products, from brake pad lining in car manufacturing to insulation and cement products for the construction industry. Asbestos exposure can cause both pleural and pulmonary disease. Asbestos-induced interstitial lung disease is referred to as asbestosis. The pathogenesis of asbestos-associated disorders was believed to be due to inspiration of fiber complexes that penetrated deep into the lungs. Several studies in the 1970s explored the response of mesothelial cells, macrophages, and alveoli to deposition of various asbestos fibers.42,43 More recent research has shed more light on asbestosis-mediating mesothelial cells, leading to release of reactive oxygen species that lead to translocation of high-mobility group box 1 (HMGB-1) protein, a driving force of oncogenesis. HMGB-1 has served as an important focus of malignant mesothelioma and has been proposed as a possible biomarker for diagnosis and a target for treatment.44 Several asbestosis-related diseases have been noted, such as benign pleural effusions, interstitial lung disease pattern secondary to asbestos, bronchogenic carcinoma, and mesothelioma.13 The risk appears to be higher for workers exposed to amphibole fibers than chrysotile fibers. The question of whether asbestos exposure alone or asbestosis represents the risk factor for lung cancer remains an area of debate, although the latter has been shown to be more important.45 In a prospective cohort study, asbestos exposure had an RR of 3.49 (95% CI 1.69-7.18) compared to the non-exposed group after adjusting for several factors, including age, smoking, and other occupational exposures.46 Asbestos and cigarette smoking are both independent causes of lung cancer but in combination have a multiplicative effect. There is also a dose- and duration-dependent increase in lung cancer risk to asbestos exposure.11 The RRs for lung cancer with asbestos exposure alone and cigarette smoking alone are 6-fold and 11-fold, respectively, but with exposure to both, the risk is increased 59-fold.47 The United States has taken extensive measures over the last 50 years to limit any use of asbestos given the increasing knowledge about its adverse health effects. The Occupational Safety and Health Act of 1970 was the first US congressional act to regulate and limit work exposures to asbestos. This regulatory power was extended by the EPA in 1976 with the Toxic Substances Control Act, allowing the EPA to monitor how chemicals were manufactured and used in various products. Additionally, asbestosis is listed as a type of air pollution in the Clean Air Act given concern for airborne fibers that can lead to respiratory issues. In the Asbestos Hazard Emergency Response Act (AHERA) of 1986, Congress forced schools to have regulations for limiting asbestos materials given unintended exposures to children. To date, there is no full ban on asbestos products. Most states have their own legislation on asbestos regulation, and most follow recommendations from the EPA’s National Emission Standards for Hazardous Air Pollutants. Such regulations include how to dispose of asbestos-containing products when doing residential demolition or renovations and how to handle asbestos-contaminated materials. The radon 222 is a gaseous decay product of elements (radium 226 and uranium 238) found ubiquitously in soil and rocks. Radon can accumulate in enclosed areas, such as mines or houses, and dissolve in groundwater. Radon is an established human lung carcinogen. It emits alpha particles that cause DNA damage in respiratory epithelium.48,49 Although radon exposure in uranium mine workers led to our understanding of its association with lung cancer, there are significant differences between the conditions of exposure in mines and those in houses. These differences include the relative proportion of radon itself to its decay products, respiratory rate, and particle size distributions.13,50 These differences may complicate extrapolation data from miners’ radon lung cancer risks to residential settings, but there have been studies that confirm generalizability of data from miners to the general population. In a combined analysis of 7 case-control studies from North America, the estimated risk of lung cancer was 11% higher after exposure to residential radon. The estimated OR after exposure to radon at a concentration of 100 Bq/m3 (2.7 pCi/L) in the exposure time window 5 to 30 years before the index date was 1.11 (95% CI 1.00-1.28). Among cases, 38% were diagnosed with adenocarcinoma, 22% with squamous cell carcinoma, and 16% with small/oat cell carcinoma.51 These findings were similar to the meta-analysis of 13 European studies.52 The RR was found to be time dependent and decreased when more time had elapsed since the last exposure. Long-term exposure yielded a greater risk than did short-term exposure, irrespective of the rate of exposure.53 The National Research Council has estimated that residential radon may account for 10% to 15% of the lung cancer burden in the United States. The average level of radon in homes in the United States is 1.3 pCi/L, and the average level outside is 0.4 pCi/L. Radon is potentially harmful in poorly ventilated structures. The EPA estimates that exposure to a radon level of 4 pCi/L has the lifetime risk of lung cancer death of 7 per 1,000 in never-smokers compared with 62 per 1,000 for ever-smokers. Approximately, 15,000 to 22,000 annual lung cancer deaths in the United States are related to radon, but only 10% of deaths occur among non-smokers.54 A map of high-radon areas in the United States is shown in Figure 3-2. Figure 3-2. Radon distribution in the United States, US Geological Society. Generalized radon potential of United States. (United States Geological Survey; 1995. https://www.epa.gov/radon/find-information-about-local-radon-zones-and-state-contact-information. Accessed August 21, 2020.) The EPA recommends taking action to remediate the residential radon level at or above 4 pCi/L and to consider remediation for levels between 2 and 4 pCi/L. An estimated 1 in 15 US homes has radon levels at or above this EPA action level. Lowering radon levels below the EPA action level has been estimated to decrease 2%-4% of lung cancer mortality.54 The cost-effectiveness analyses of radon control strategies in the United States have been shown to reach the threshold level only if residential high-risk patients like smokers are engaged in testing and remediation.50 Silica is the most abundant mineral on Earth. It exists in amorphous and crystalline forms. The latter is associated with various pulmonary diseases referred to as silicosis. The IARC classified crystalline silica as a human carcinogen in 1997. In the largest meta-analysis of 85 studies, the pooled standardized mortality ratio (SMR) and standardized incidence ratio (SIR) were 2.32 (95% CI 1.91-2.81) and 2.49 (95% CI 1.87-3.33), respectively, in silicosis and 1.78 (95% CI 1.07-2.96) and 1.18 (95% CI 0.86-1.62), in non-silicosis patients, respectively. A positive exposure-response relation was found between cumulative silica exposure and risk of lung cancer.55 Based on this evidence, the US Occupational Safety and Health Administration (OSHA) lowered the occupational exposure limit for crystalline silica from 0.1 to 0.05 mg/m3. Concomitant smoking has an additive effect on lung cancer risk from silica exposure. In a large cohort study, risk of lung cancer death among smokers exposed to silica (HR 5.07, 95% CI 3.41-7.52) was higher compared to non-smokers (HR 1.60, 95% CI 1.01-2.55) when exposed to the same cumulative dose of silica (>1.12 mg/m3).56 Occupational exposures to several metals, like arsenic, beryllium, cadmium, chromium, and nickel, are also known to increase lung cancer risk.57 Several previously published observational studies have reported beneficial effects of the dietary factor on lung cancer. For example, antioxidants like vitamins A, C, and E are shown to have cancer-protective effects. Unfortunately, these effects were not observed in several randomized controlled trials. In a prospective epidemiological study in 1954, the dietary intake of beta-carotene was associated with a lower 19-year incidence of lung cancer in middle-aged men.58 In a case-control study, the RRs by vitamin A intake quartiles (lowest to highest) were 1.8, 1.8, 1.0, 1.0 (p for trend = .001) for men. The effect was not statistically significant for women.59 At least 2 large randomized double-blind control trials, Beta-Carotene and Retinol Efficacy Trial (CARET) and Alpha-Tocopherol, Beta Carotene Cancer Prevention (ATBC), failed to show such a beneficial effect. Unexpectedly, the mortality rate and incidence of lung cancer in the experimental arm was higher.60–62 A large cohort study from the Netherlands has reported the protective effect of a diet rich in fruits and vegetables on lung cancer incidence. Protective effects of fruits and vegetables were stronger in current than in former smokers.63 In the National Institutes of Health–AARP Diet and Health Study, total fruit and vegetable intake was not associated with lung cancer incidence, although higher consumption of certain botanical subgroups (rosaceae, convolvulaceae, and umbelliferae) had a significant inverse relation to cancer risk in men only.64 There are reports of other dietary items, like saturated fats, dairy products, and smoked and salted meats, increasing the risk of lung cancer.65–67 Despite the inconsistent results of trials and epidemiological studies, the current recommendation by the American Cancer Society https://www.cancer.org/cancer/lung-cancer/causes-risks-prevention/prevention.html is to have a balanced dietary intake and avoid overindulgence in vitamins and other dietary supplements. The role of infection as a risk factor for lung cancer remains debatable. Lung cancer is the most common non–AIDS-defining malignancy in patients with HIV infection. With the introduction of highly active antiretroviral therapy (HAART), AIDS-related mortality has significantly dropped, but there has been an accompanying increase in lung cancer–related deaths, accounting for 30% of all cancer deaths and 10% of all non–HIV-related deaths.68 There is no evidence that HAART therapy directly led to this increased risk. Although smoking is more prevalent among individuals with HIV, the risk of lung cancer remains significantly high even after adjusting for smoking status.69 Among 2,086 AIDS patients, HIV infection was associated with increased lung cancer risk (HR 3.6, 95% CI 1.6-7.9) after adjusting for age, sex, smoking status, and calendar period.70 Although the not well established but frequent co-infection with oncogenic viruses (Epstein-Barr virus, human herpesvirus, or human papilloma virus [HPV]), the direct effect of HIV virus or prolonged immunosuppression may be the cause of the excess risk of lung cancer in this population.13 Lung cancer in an individual with HIV/AIDS tends to present in younger patients, more advanced stage, and has significantly reduced overall survival.68,71 HPV has been implicated in various cancers, such as genital, anal, and oropharyngeal cancers, but studies regarding its etiologic role in lung cancer have been inconsistent. The possible involvement of HPV in bronchial squamous cell lesions was first suggested in 1979 by Syrjanen, who described epithelial changes in bronchial carcinomas closely resembling those of established HPV lesions in the genital tract, such as exophytic and flat condyloma.72 The highly oncogenic HPV types associated with lung cancer include 16, 18, 31, 33, and 35. There is inconsistency in the reported prevalence of infection by HPV in patients with lung cancer in different countries, with racial and geographic variations. One study on lung biopsies from Taiwanese patients showed a significant difference between HPV 16 and HPV 18 infection in lung cancer versus non-cancerous samples (54.6% vs 26.7%).73 A study in patients from Wuhan showed a different incidence (27.7% vs 5.9%) for HPV 16 and HPV 18 in cancerous and non-cancerous lung samples.74 On the other hand, in Western Europe, studies with a large number of patients failed to show an etiologic role for HPV in lung cancer.75,76 Variability in the reported number of HPV-positive lung cancer may be explained by several factors, such as environmental variables, high-risk behavior, genetic susceptibility, and methodologic approaches with varying sensitivity and specificity for HPV identification.77 In a recently published meta-analysis, HPV infection was associated with cancer of lung; the pooled OR was 3.64 (95% CI 2.60-5.08).78 It would be interesting to see if the HPV vaccine has had any impact on the incidence of lung cancer. There is some evidence that certain chronic non-malignant lung diseases may be independent risk factors for lung cancer. Chronic obstructive pulmonary disease (COPD) has been reported to have the strongest risk factor for lung cancer. Although tobacco is the primary cause for both COPD and lung cancer, studies have shown increased lung cancer risk in patients with COPD independent of smoking status. One study showed a significantly higher prevalence of COPD in patients with lung cancer (50% vs 8%) compared to those in a randomly recruited control group (OR 11.6, p < .0001) after adjustment for age, sex, and smoking exposure.79 COPD has been suggested to be an independent risk factor for lung cancer by increasing oxidative stress, chronic inflammation, defective DNA repair mechanisms, and increased cellular proliferation.80 A large retrospective study has shown cancer-protective effects of inhaled corticosteroids in patients with COPD, which further supports the theory of chronic inflammation leading to lung cancer.81 Patients with idiopathic pulmonary fibrosis (IPF), regardless of smoking status, have been reported to have up to an 8-fold increase in risk of cancer compared to controls.82 The mechanism of this increased risk remains unclear. A study from the United Kingdom compared 1,064 patients with IPF to 4,238 matched controls found a significant in increase in the incidence of lung cancer (rate ratio 4.96; 95% CI 3.00-8.18) after adjusting for age, gender, and smoking status.83 Besides the confounding effects of smoking, the fibrosis might itself lead to carcinogenesis by the occurrence of atypical or dysplastic epithelial changes.82,84 In a case-control study, α1-antitrypsin deficiency carriers had a higher risk of lung cancer when compared to unrelated non-carriers (OR 1.7, 95% CI 1.2-2.4) after adjusting for tobacco use and COPD diagnosis.85 The genetic risk factors for lung cancer are poorly elucidated. This is supported by the fact that only a fraction of tobacco smokers develop lung cancer, and positive family history is an independent risk factor. In a meta-analysis of 41 published cohort and case-control studies, an affected family member led to a significantly higher risk of lung cancer (RR 1.72, 95% CI 1.56-1.88). The association was only slightly weaker among non-smokers (OR 1.4, 95% CI 1.17-1.68). A positive family history of lung cancer in 2 or more relatives was associated with higher risk (OR 3.6, 95% CI 1.56-8.31).86 In a retrospective study of lung cancer patients who were never smokers, 18% of the patients had a family history of lung cancer. In a subgroup of patients with lung cancer that has an EGFR mutation or ALK translocation, 23% and 12% of the patients had a family history of lung cancer, respectively.87 The familial incidence of lung cancer may be caused by similar environmental factors or an inherited susceptibility, but teasing out the independent effect of the latter may be difficult. The genetic factors associated with tobacco-induced lung cancer have been extensively investigated. Large-scale genome-wide association (GWA) studies have identified several lung cancer–susceptible genes. Some of the notable genes are on chromosome 5p15.33, 6p21, and 15q24-25.1. The 15q25 region contains 3 nicotine acetylcholine receptor subunit genes, and its polymorphism has been associated with nicotine dependence. The 5p15.33 region is associated with risks specifically for lung adenocarcinoma. The 6q23-25 and 13q31.3 regions were also identified by GWA studies as being associated with risk for lung cancer, particularly in never-smokers.88,89 Further studies are required to understand the individual risk of lung cancer based on genetic factors. Radiation can cause detrimental effects to the exposed tissue by direct damage, generation of free radicals, and inflammation. Radiation therapy (RT) for other cancers like breast cancer and Hodgkin lymphoma increases the risk of secondary lung cancer. In an observational study, breast cancer treated with RT had a higher risk of a second primary lung cancer (2.25% vs 0.23%) compared to patients who did not receive RT (HR 10.078, 95% CI 3.713-27.351).90 One study of long-term complications of Hodgkin lymphoma reported a mean RR of 2.6-7.0.91 With improvement in radiation techniques, the risk of second primary lung cancer may not be this prominent. In the analysis of the Italian COSMOS lung cancer screening trial, an estimated lifetime attributable risk of lung cancer ranged from 5.5 to 1.4 per 10,000 people after 10 years of low-dose computed tomographic screening.92 This risk has been deemed acceptable as a trade-off to significant reduction in lung cancer mortality in high-risk patients who are currently recommended for lung cancer screening. There is evidence for increased lung cancer risk in industrial radiation workers, especially those who process plutonium and may inhale radioactive particles. There does not seem to be an increased risk of lung cancer in health care workers who perform fluoroscopic procedures.93 A list of common causes of lung cancer is shown in Table 3-1. TABLE 3-1 Causes of Lung Cancer, Estimates of Relative Risk
3
ETIOLOGY OF LUNG CANCER
RISK FACTORS
Tobacco
Passive Smoking
Electronic Cigarettes
Cannabis
ENVIRONMENTAL AND OCCUPATIONAL FACTORS
Air (Outdoor) Pollution
Indoor Pollution
Asbestos
Radon
Other Occupational Exposures
DIETARY
INFECTION
CHRONIC LUNG AND AIRWAY DISEASE
GENETIC RISK FACTORS
RADIATION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree