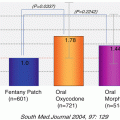
Fig. 1.1
Number of deaths due to oral and pharyngeal cancers by developed country (per 100,000 population). Increase is observed only in Japan among developed countries and this is a disturbing fact
Clinical statistics obtained in our department and measures for prevention of oral cancer that we addressed are presented, as well as epidemiology is discussed in this article.
1.2 Frequency of Oral Cancer
The number of cancer patients as well as patients with oral cancers is increasing with the advent of a super-aging society in Japan. The incidence rates of oral cancer are different by ethnic, country, region, lifestyle, and practice.
Although an exact nationwide survey on oral cancers has not been conducted yet, the numbers of death from oropharyngeal cancers per 100,000 of population in males and females were 2.4 and 1.3, 5.1 and 2.9 in 1975 and 1995, respectively, and are estimated to increase to 8.6 and 5.2 by 2015 according to the Annual Report on Health and Welfare [2]. Treatment performance has also been improved during these years; however, the increase of death from cancers largely exceeds the treatment performance. This means the development of cancers also increases. The incidence of oral cancers in Japan is as described in the above section [2–4], and this number corresponds to about 1 % of the total cancer number and 40 % of the total number of head and neck cancers. Therefore, it is considered that such steady increase was caused by an increase of super-aging people [6–13].
Age-adjusted prevalence of oral cancer is highest in 60’s similar to other cancers, and the male-to-female ratio is 3:2, which is higher in males than in females. According to nationwide statistics of Japanese Society of Oral and Maxillofacial Surgeons on oral cancers in 2002 [14], of 1777 patients, male patients were 1,051 (59.1 %) and female patients were 726 (40.9 %). By age groups, 50s patients were 323 (18.1 %), 60s patients were 471 (26.5 %), and 70’s patients were 517 (29.1 %), and patients of 50 years old and older account for 80 % of the total patients. In the present day seeing the super-aged society, it is estimated that the number of old–old patients would increase furthermore. Although it was previously considered that the development of oral cancer in 30’s and younger is rare, increase in 20’s patients is reported in these years. Effects of lifestyle, living environment, chemical factors, viruses, and bacteria are considered. Clinical characteristics with recently 10-year oral cancer patients in our department are shown in Table 1.1 and Figs. 1.2, 1.3, and 1.4.
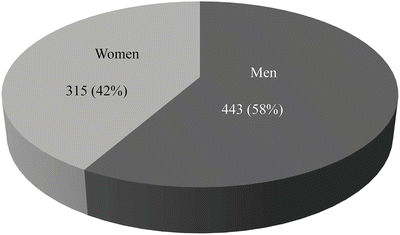
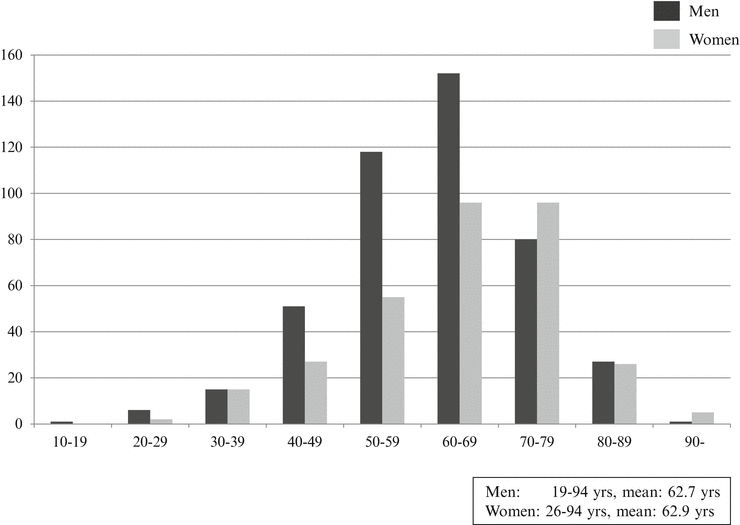
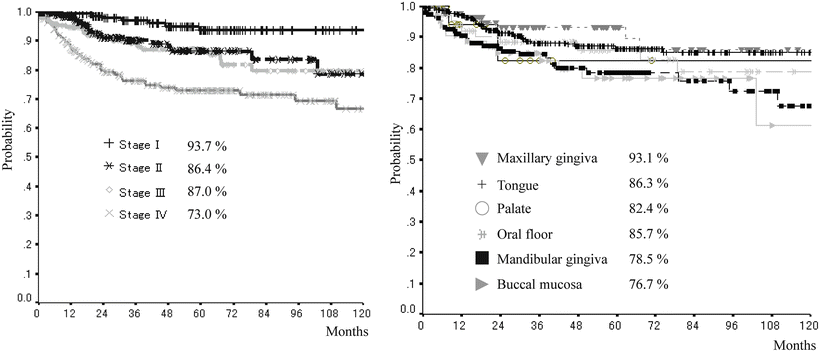
Table 1.1
Oral cancer patient characteristics in our department (2003/1–2012/12, n = 348)
Patient characteristics | n = 348 |
|---|---|
Age, mean (range) | 62.9 (21–94) |
Sex, men/women | 188/160 |
Performance status | 0–2 |
Primary site (%) | |
Tongue | 183 (52.6) |
Mandibular gingiva | 68 (19.5) |
Maxillary gingiva | 38 (10.9) |
Buccal mucosa | 26 (7.5) |
Oral floor | 22 (6.3) |
Palate | 9 (2.6) |
Others | 2 (0.6) |
Histology (%) | |
Well | 102 (29.3) |
Moderate | 36 (10.3) |
Poor | 8 (2.3) |
Early | 36 (10.3) |
Unknown | 166 (47.7) |
Mean follow-up, month (range) | 46.3 (4.4–123.2) |
T-stage (%) | |
1 | 113 (32.5) |
2 | 151 (43.4) |
3 | 36 (10.4) |
4 | 48 (13.8) |
N-stage (%) | |
0 | 209 (60.1) |
1 | 77 (22.1) |
2a | 8 (2.3) |
2b | 33 (9.4) |
2c | 21 (6.0) |
Stage (%) | |
I | 103 (26.5) |
II | 94 (24.2) |
III | 74 (19.1) |
IV | 77 (19.8) |

Fig. 1.2
The incidence according to the men and women with oral squamous cell carcinoma in our department. The male-to-female ratio is 3:2, which is higher in males than in females

Fig. 1.3
The age distribution according to the men and women with oral squamous cell carcinoma in our department. Age-adjusted prevalence of oral cancer is highest in the 1960s similar to other cancers

Fig. 1.4
The 5-year survival rate according to stage and site of the oral squamous cell carcinoma in our department
The incidence of oral cancers is high in countries with a higher rate of both smoking and drinking habit [10, 15], especially high in south Asian countries. It is considered that a habit of chewing tobacco such as betel nut highly contributes to this tendency, and it is estimated that the incidence rate of oral cancer is 0.5–5 % and the number of patients with oral cancer reaches 2.5 million in India [16–19]. Mortality rate from oropharyngeal cancer in Japan is lower than France and Italy, and this is considered because this is largely affected by food and life habitat [5].
Cancer registry is increasingly becoming popular, but still inadequate. Establishment of nationwide cancer registry in consideration of the Private Information Protection Law would be required.
1.3 Favorite Site
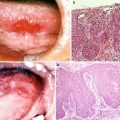
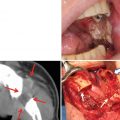
Favorite site of oral cancer is different depending on the race and lifestyle. We describe about frequency of oral cancer occurrence by sites in Japan in this section. The frequency of oral cancer occurrence by sites is different depending on the ethnic, country, region, lifestyle, and practice. According to a tally by the Japanese Society of Oral and Maxillofacial Surgeons in 2002, the occurrence frequency of oral cancer (n = 1,784) by sites was highest in the tongue and accounted for 40 % of the entire oral cancers [14], followed by the mandibular gingiva (20.3 %), maxillary gingiva (12.0 %), buccal mucosa (10.3 %), oral floor (9.2 %), maxillary antrum, and palate in Japan [5]. In the USA, the frequencies were reported as the tongue (35.2 %), oral floor (28.0 %), mandibular and maxillary gingiva (10.4 %), hard palate (8.9 %), and buccal mucosa (2.9 %) [10]. Eighty percent of tongue cancer tends to occur at the lingual border, and it is exceptionally rare to occur at the apex or back of tongue. For gingiva, research has still not shown that the occurrence frequencies are different between the maxilla and mandible. The occurrence frequency in our department was also in the order of the tongue, mandibular gingiva, oral floor, buccal mucosa, maxillary gingiva, palate, and lips, in which percentages almost corresponded to the statistics published from other facilities (Table 1.1). Characteristics by sites of occurrence are summarized as below:
1.
Tongue cancer (Fig. 1.5a)
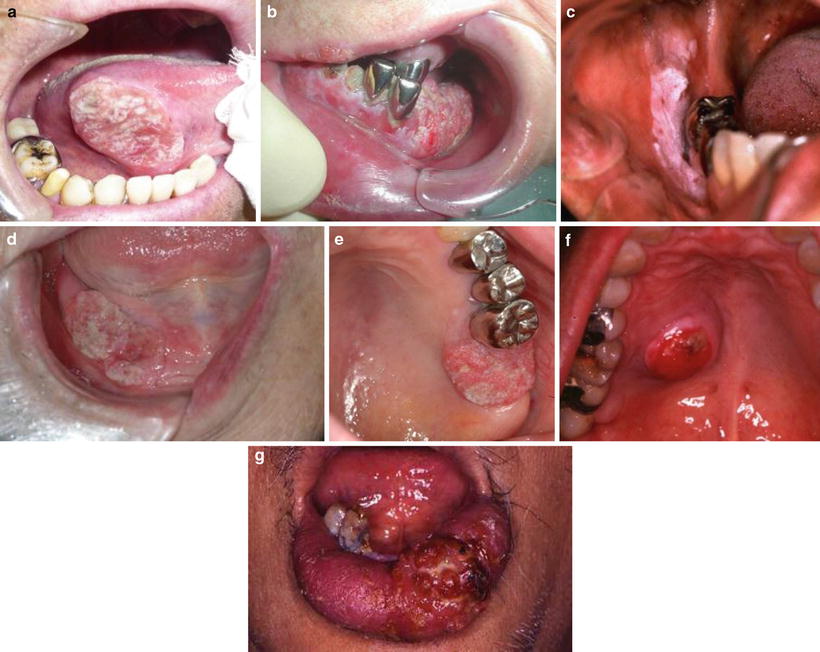

Fig. 1.5
Favorite site of oral cancer. (a) Tongue cancer. (b) Mandibular gingival cancer. (c) Buccal mucosa cancer. (d) Oral floor cancer. (e) Maxillary gingival cancer. (f) Palate cancer. (g) Lower lip cancer
(a)
Tongue cancer accounts for 40 % of the entire oral cancers and makes up the majority of the oral cancers.
(b)
Tongue cancer occurs more commonly at the lingual border or inferior surface of tongue and occurs only infrequently at the apex or back of tongue.
(c)
Advanced tongue cancer spreads over the oral floor and tongue base and causes adhesion, lingual movement disorder, dysmasesis, dysphagia, dysarthria, and trismus, and it results in respiratory distress when it progresses to the pharyngeal region.
2.
Mandibular gingival cancer (Fig. 1.5b)
(a)
Mandibular gingival cancer accounts for 20 % of the oral cancers and occurs in the next highest number after tongue cancer.
(b)
It is often detected through symptoms such as inadaptation of denture, swelling or ulcer formation of gingiva, or tooth movement.
(c)
It is often treated with misdirected therapy including tooth extraction, anti-inflammation treatment, and adjustments to denture without aim based on the diagnosis of periodontal diseases and stomatitis.
(d)
Mandibular gingival cancer is likely to cause destruction and absorption of the mandibular bone in relatively early stage because the tumor becomes infiltrated along the periosteum. Infiltration is categorized into three types, pressure type, invasive type, and moth-eaten type based on its characteristics.
3.
Buccal mucosa cancer (Fig. 1.5c)
(a)
The frequency of buccal mucosa cancer is only about 10 % in Japan, but is highest, about 50 %, in India. It is believed that the reason of such a high rate in India is caused by betel nut and chewing tobacco, which are key carcinogenic factors.
(b)
Buccal mucosa cancer occurs more commonly in buccal mucosal surface facing to the molar tooth region and in the distomolar region.
(c)
Most of the cancers are well-differentiated type and often associated with leukoplakia.
4.
Oral floor cancer (Fig. 1.5d)
(a)
The frequency of oral floor cancer is about 10 % and relatively low. Patients become aware of mass formation associated with ulcer and induration of the oral floor in many cases.
(b)
Oral floor cancer infiltrates into the opening or duct of the submandibular gland and may be associated with excretory disturbance of saliva or swelling of the submandibular gland.
(c)
Oral floor cancer can spread to the tongue and gingiva or adhere to periosteum of the jawbone or infiltrate into suprahyoid muscles, which form the oral floor, relatively early in the course because the oral floor is close to the tongue, gingiva, and mandible.
(d)
Patients have marked pain and a feeling of strangeness during eating and talking and also easily develop inadaptation of denture.
5.
Maxillary gingival cancer (Fig. 1.5e) and maxillary sinus cancer
(a)
Maxillary cancer consists of maxillary gingival cancer, which develops from the gingiva, and maxillary sinus cancer, which occurs primarily in the maxillary sinus mucosa. The frequency of oral floor cancer is about 10 % and relatively low.
(b)
Subjective symptoms of maxillary gingival cancer include swelling, ulcer, and pain, but few tooth pain occurs. When advanced, the cancer infiltrates and destroys the buccal and palatine mucosa, as well as the nasal cavity and maxillary sinus floor.
(c)
Subjective symptoms of maxillary sinus cancer are mainly nasal symptoms, such as nasal congestion, rhinorrhea, and nasal bleeding; oral symptoms, such as tooth pain, tooth movement, and swelling of the palate; and swelling of the cheek. If the cancer spreads into the orbit, exophthalmos, double vision, and visual impairment occur.
6.
Palate cancer (Fig. 1.5f)
(1)
The frequency of palate cancer is about 2 % and low. The site of onset is the hard palate by gingiva in general; however, the cancer crosses over the midline or results in absorption and destruction of the soft palate, gingiva, or palatal bone in advanced cases.
(b)
Subjective symptoms are mainly swelling of the palate region and followed by ulcer and pain.
7.
Lip cancer (Fig. 1.5g)
(a)
The frequency of lip cancer is about 1 % and lowest.
(b)
Lip cancer occurs more commonly in the lower lip and develops ulcers and swelling relatively early in the course.
1.4 Risk Factors and Prevention of Oral Cancer
1.
Risk factors of oral cancer
A number of risk factors of oral cancer have been reported [21, 22]. Risk factors of oral cancer reported up to the present are summarized in Fig. 1.6. Surprisingly, however, only few factors have been epidemiologically or experimentally established. In addition, it is widely recognized as an underlying concept that oral cancer is very unlikely to develop with single factor and is caused by overlapping several factors in multistages. Especially smoking and drinking are representative risk factors of oral cancer, which are epidemiologically and experimentally established.


Fig. 1.6
Risk factors of oral cancer
2.
Smoking and oral cancer
There are no other risk factors like smoking in which causal association with cancer has been clearly established. Now, tobacco could be said to be an enemy of every disease to put it in extreme terms. Smoking is a luxury item, which also has the highest causal relation with carcinogenesis in oral cancer.
The occurrence frequency of oral cancer is extremely high in Sri Lanka, India, or Taiwan as compared with Japan, and about 30 % of the entire cancer is oral cancer. It has been epidemiologically shown that such high occurrence of oral cancer is caused by a habit of chewing tobacco and betel nut (Fig. 1.7) [21]. The Declaration of Opposition to Smoking is adopted by about 40 academic societies including the Japanese Society of Oral and Maxillofacial Surgeons at the present day and supports promotion of the antismoking movement in Japan. It is indisputable that smoking is the largest factor for the prevention of oral cancer. A number of studies on carcinogenicity of smoking have been conducted since the mid-1900s, and it has been demonstrated that there are a number of substances that act as initiators or promoters in the carcinogenic process in about 4,000 kinds of chemical substances contained in cigarette smoke [23]. About 40 substances including benzopyrene and nitrosamines have been identified as carcinogenesis-related substances to date [23]. In addition, it is considered that oral cancer, pharyngeal cancer, laryngeal cancer, esophageal cancer, gastric cancer, pancreatic cancer, liver cancer, kidney cancer, bladder cancer, and uterine cancer are associated with smoking. Recently, smoking rate of Japanese is on a declining trend. However, it is pointed out that an increasing tendency is observed in only young females.


Fig. 1.7
Chewing tobacco and betel nut in Taiwan. Betel nut and chewing tobacco, which are key carcinogenic factors in buccal mucosa cancer
3.
Drinking and oral cancer
Drinking is a factor, which causation with oral cancer has been demonstrated similar to smoking. Different from tobacco, alcohol (= ethanol) itself has no carcinogenicity. However, it has been clarified in many studies that alcohol is indirectly associated with carcinogenesis. Moreover, a synergistic effect due to concomitant exposure to drinking and smoking often becomes a problem. Drinking and smoking cause exposure of carcinogenic factors to all pharynx, larynx, esophagus, and stomach in addition to the oral cavity at the same time (field cancerization); therefore, this is also related to the development of double cancer. According to our department, double cancers with upper digestive tract cancer were observed in 10.4 % of the entire oral cancer patients [24]. The carcinogenic mechanism of alcohol was poorly understood until recently in contrast to tobacco. There is no report of carcinogenesis induced by only alcohol administration in animal experiments. However, International Agency for Research on Cancer (IARC) recognized alcohol as a carcinogen because acetaldehyde, which is a metabolic product of alcohol, has carcinogenicity [25]. In other words, the carcinogenic mechanism may directly or indirectly interact with biological reactions. As direct interaction, metabolic enzymes localizing in the oral mucosa degrade alcohol, and it causes exposure of the mucosa to acetaldehyde. Homann et al. [26] reported that bacteria existing inside the oral cavity increase carcinogenic risk of oral cancer by degradation of alcohol to acetaldehyde. As indirect interaction, it is considered that effects of alcohol include effect of acetaldehyde metabolized in the liver on local mucosa, effect as a solvent of carcinogens derived from tobacco and others, decrease of metabolic function of the liver, decrease of immunological capacity due to alcohol ingestion, and lowering of nutritional status.
4.
Epidemiology of smoking and drinking
As the results of a meta-analysis of reports on a large-scale epidemiological studies, IARC concluded that smoking and drinking are distinct risk factors of oral cancer [25]. Of course, the more daily consumption of as well as longer exposure time to smoking and drinking result in increase of carcinogenic risk. Accordingly, an index that takes account of them has an important implication. Brinkman index and Sake index shown in Fig. 1.8 are widely used to express relationship between the amount and duration of smoking and drinking with oral cancer. Sake index that is 60 or higher and Brinkman index that is 1,000 or higher are defined as the risk zones (Fig. 1.8). However, pack-years is used as a new smoking index instead of Brinkman index in recent years. This index is calculated by dividing Brinkman index by 20.
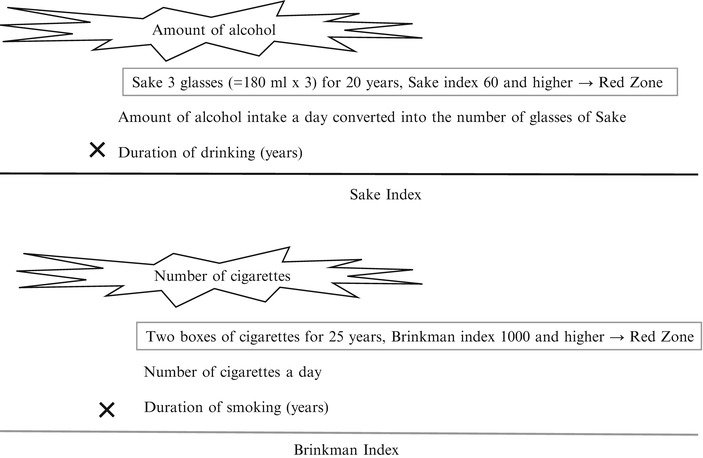

Fig. 1.8
Oral cancer risk and Sake and Brinkman indices. Sake index that is 60 or higher and Brinkman index that is 1,000 or higher are defined as the risk zones
Based on the above findings, we conducted a case–control study in 191 patients with oral cancer and 121 healthy subjects with no oral mucosal diseases who visited our department [27]. The results of multiple logistic analysis on risk for the development of oral cancer are shown in Table 1.4. Risk of developing oral cancer (odds ratio) by smoking alone was 2.5, but it elevated to 4.3 in heavy smokers with Brinkman index 1,000 or higher. In addition, odds ratio of drinking alone was 4.5; however, it elevated to 10.4 in heavy drinker with Sake index 60 or higher. When subjects have a habit of smoking and drinking, the risk of developing oral cancer resulted in 4.8, which was higher than smoking or drinking alone. In other words, it is considered that the risk of oral cancer is high in people with Brinkman index 1,000 or higher and Sake index 60 or higher and especially high in people who have both smoking and drinking habits.
5.
Genotypic analysis of metabolic enzymes related to smoking and drinking
Carcinogens accumulate in the body through tobacco use or alcohol ingestion and then are metabolized and detoxified. Recently, it is revealed that there are differences in individual’s metabolic capability due to genetic variant of metabolic enzymes. In other words, it is speculated that the same loading from smoking and/or drinking may cause different degrees of carcinogenic risk in individuals because metabolic and detoxification capabilities against carcinogens vary considerably from individual to individual. Accordingly, we conducted an analysis of genes related to smoking and drinking in 127 patients with oral cancer and 33 healthy subjects with smoking and drinking habits, who preliminarily gave informed consent (Table 1.2) [27]. As a smoking-related enzyme, gene polymorphism of glutathione S-transferase M1 (GSTM1), which is an enzyme degrading benzopyrene in tobacco, was identified and analyzed with a multiple logistic analysis (Table 1.3) [27]. As a result, the risk of developing oral cancer in smokers with GSTM1 gene mutation was 2.5 times higher. Then aldehyde dehydrogenase 2 (ALDH2), which is an enzyme that degrades acetaldehyde, was identified and analyzed with a multiple logistic analysis. As a result, the risk of developing oral cancer in individuals with drinking habit with ALDH2 gene mutation (hetero deletion) was 2.9 times higher. Furthermore, an Italian research team recently conducted a meta-analysis of papers on ALDH2 gene polymorphism, including our report, and concluded that carcinogenic factor of drinking-related head and neck cancers is acetaldehyde [28]. Moreover, they also reported that higher alcohol consumption causes higher carcinogenic risk in ALDH2-deleted individuals [28].
Table 1.2
Oral cancer risk in smoking and drinking-related metabolic genes
Odds ratio (95 % confidence interval) | |
|---|---|
Patients with GSTM1 defect | 2.5 (1.6–5.4) |
Patients with ALDH2 hetero defect | 2.9 (1.1–7.8) |
Table 1.3
The risk of developing oral cancer in smoking and drinking
Odds ratio (95 % confidence interval) | |
|---|---|
Smoking | 2.5 (1.1–5.6) |
B.I. 1,000∽ | 4.3 (1.6–11.5) |
Drinking | 4.5 (2.5–8.1) |
S.I. 60∽ | 10.4 (3.6–29.4) |
Smoking + drinking | 4.8 (1.8–13.0) |
As mentioned above, the polymorphism pattern of metabolism-related genes may contribute to the identification of risk factors of oral cancer because the polymorphism pattern varies from individual to individual. New biomarkers would be discovered in the future, and the development of tailor-made prevention may be expected in this area.
6.
Virus infection and oral cancer
Several types of carcinogenesis caused by virus infection have been reported. For example, it is famous that hepatitis B virus and hepatitis C virus cause liver cancer and is also well known that adult T-cell leukemia (ATL) is caused by RNA virus (retrovirus). In the head and neck region, EB virus belonging to herpesvirus group causes Burkitt’s lymphoma. In addition, it is reported that human papillomavirus (HPV) is associated with the development of oral cancer [29]. Recently HPV is watched with interest as the cause of oral cancer especially in young individuals with no risk factors of smoking and drinking.
7.
Age and oral cancer
Japan is becoming an unprecedented aging society with a falling birthrate in the world with progress of super-aging society and lowering of birthrate in these years. It is reported that oral cancer appears most frequently at 50 years old and older. However, it is considered that recent increasing tendency is due to the increasing number of the elderly.
8.
Oral cancers in high-risk females (Figs. 1.9, 1.10, and 1.11)
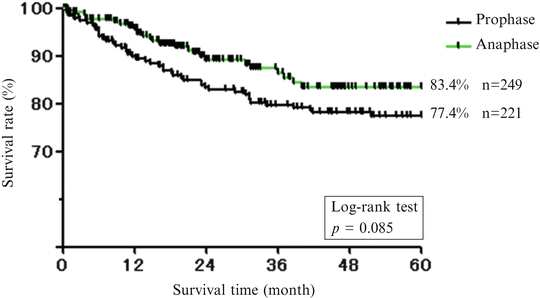
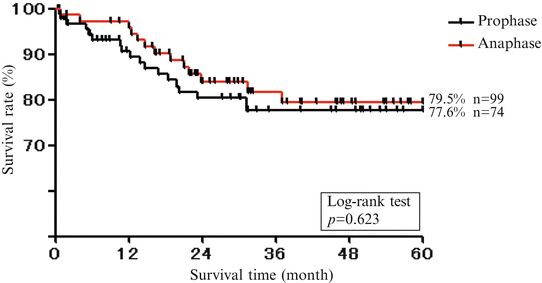
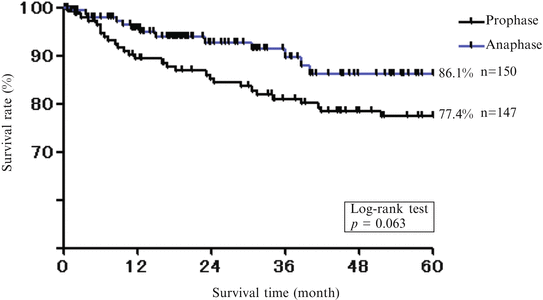

Fig. 1.9
Five-year survival rate (whole men and women). The first 10 years and later 10 years were analyzed separately

Fig. 1.10
Five-year survival rate (women). Five-year survival rate was lower in females than males

Fig. 1.11
Five-year survival rate (men). Five-year survival rate was lower in females than males
In general, it is believed that oral cancers are often found in middle-aged males and less frequent in females. It is presumed that this is greatly associated with having much stress from work in addition to lifestyle habits such as smoking and drinking. However, increase of female patients is recently being seen as a problem. There are more than a small number of female patients with no smoking/drinking history and no clear carcinogenic cause. There is a report that HPV, which is a cause of cervical cancer, is associated with carcinogenesis; however, concrete conclusion is still not obtained. Especially female patients need attention to aesthetic recovery in addition to functional aspect sufficiently taking into account the social background and living environment.
We compared treatment results of males and females with oral cancer in recent two decades (the first 10 years and later 10 years were analyzed separately) in our department (Fig. 1.9). As a result, 5-year survival rate was lower in females (Fig. 1.10) than males (Fig. 1.11), and it was noted that the number of recurrence and metastasis was increased in females diagnosed with early cancer.
9.
Young patients and oral cancer
It has been known that oral cancer often occurs in males of 60 years and older; however, recently it is pointed out that the rate of oral cancer in young individuals and females is increasing [30–32]. There is no strict definition of “young individuals,” but many reports use 40 years old as a benchmark [30–36]. Our university has also examined for 25 years from 1987 to 2012, and the rate of oral cancer in young individuals below 40 years old was 37/758 cases (5.0 %), which was almost the same rate as reported from other facilities. On the other hand, statistically significant increase was observed in annual changes in the rate of cancer in young individuals (below 40 years old) (Fig. 1.12).
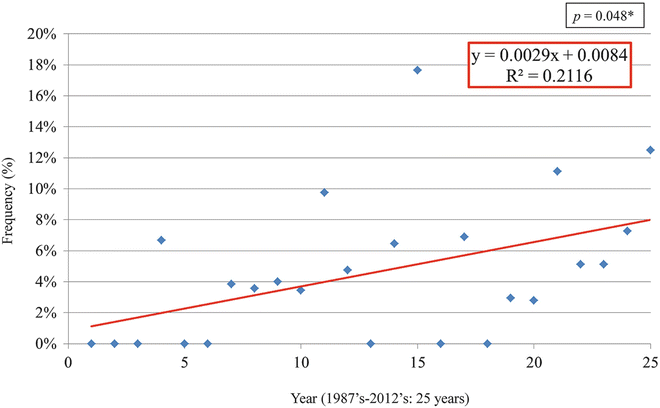

Fig. 1.12
Annual change in the rate of cancer in young patients (under 40 years old). Statistically significant increase was observed in annual changes in the rate of cancer in young individuals (below 40 years old) (p = 0.048)
Critical causes include age, sex, smoking, alcohol consumption, virus, and mechanical factors such as poorly fitted prosthesis and sectorial tooth, and genetic abnormality. However, critical causes for young individuals are still unclear [32–34]. Many of the patients in our university had no smoking/drinking history; therefore, it was considered that mechanical factors including odontoparallaxis and malposition of a tooth might cause the cancers.
In addition, it has been known that male-to-female ratios of oral cancer in young individuals come closer to 1 [34]. The majority of primary sites were the tongue [30–34], and well-differentiated squamous cell cancers are often observed in terms of pathological appearance [32]. The male-to-female ratio in our university was 1.38, and a decrease in age was also observed as compared with a group of 40 years and older. Furthermore, the rate of primary sites was significantly high in the tongue, 76.3 %. For the degree of differentiation, the rate of well-differentiated cancers was 39.5 % (Table 1.4).
Table 1.4
Characteristics of young patients with oral cancer in our department (1987/1–2012/12, n = 38)
Patient characteristics | Young (under 40 years) n = 38 |
|---|---|
Age, median (range) | 34 (19–40) |
Sex, men/women (sex ratio) | 22/16 (1.38) |
Primary site (%) | |
Tongue | 29 (76.3) |
Maxillary gingiva | 5 (13.2) |
Mandibular gingiva | 1 (2.6) |
Others | 3 (7.9) |
Follow-up median month (range) | 35.8 (3.2–268.8) |
Differentiation (%) | |
Well | 15 (39.5) |
Others | 23 (60.5) |
Stage (%) | |
I | 11 (28.9) |
II | 9 (23.7) |
III | 9 (23.7) |
IV | 9 (23.7) |
For prognosis in young individuals, there is not yet a unified view: some reports concluded that it was better in young individuals [34–36], some other reports concluded that it was almost the same [30, 32], and others concluded that it was poor in young individuals [33]. Results in our university indicated that young individuals had good outcome: 5-year overall survival rate was 94.3 %; 5-year relapse-free survival rate was 88.2 %. However, there was no statistically significant difference as compared with a group of 40 years and older, and the rates were equivalent (Fig. 1.13). It is anticipated that pathogenic mechanism would be understood in the future.
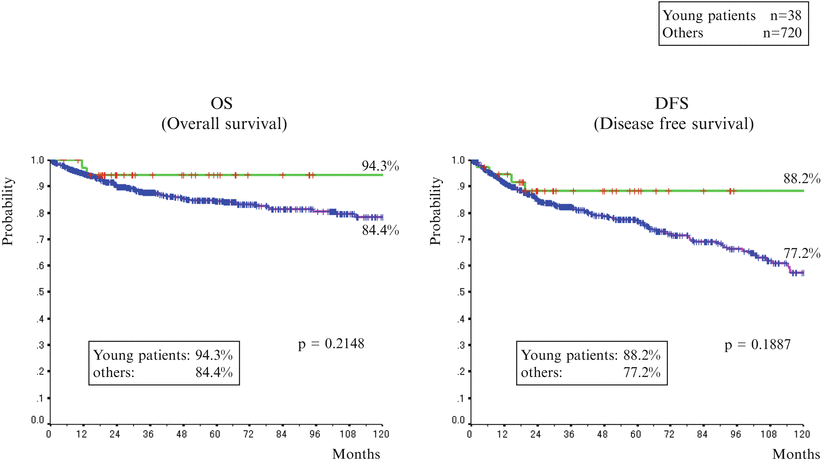

Fig. 1.13
The comparison between survival rates with young patients and others (1987/1–2012/12: 25 years). Five-year overall survival rate was 94.3 %; 5-year relapse-free survival rate was 88.2 %. However, there was no statistically significant difference as compared with a group of 40 years and older, and the rates were equivalent
10.
Oral cancer and prevention
(a)
Three steps for cancer prevention
A concept, which is adopted from the concept of natural disease prevention proposed by Leavell and Clark for cancer prevention, is the following:
Primary prevention: To prevent the onset of cancer by reducing and eliminating risk factors of health issues
Secondary prevention: To implement early detection and early treatment of cancer
Tertiary prevention: To conduct rehabilitation to promote early return to society without increasing in severity of the cancer as much as possible and to prevent recurrence
(b)
Eight items for cancer prevention (Fig. 1.14)


Fig. 1.14
“Evidence-based cancer prevention” proposed by the National Cancer Center
The National Cancer Center proposed “Evidence-based cancer prevention” in 2005. This is developed based on past enormous amount of statistics and experimental data and based on scientific evidence. This proposal concluded that about 60 % of the entire cancers (30 % by tobacco cessation and further 30 % by devices of dietary habits and others) could be prevented by implementing these eight items. This is beneficial information for us oral surgeons and also results in enlightenment of patients.
(c)
Summary of prevention
Measures against smoking, drinking, diet, and infectious diseases are important for the primary prevention of oral cancer, that is to say, to avoid becoming oral cancer. Now, most of the patients with cancer and their families, or more widely the people, are increasingly demanding for cancer care. Therefore, it is most important to achieve the target of oral cancer prevention through cooperation among the following three bodies: (a) patients with cancer, their families, and people; (b) healthcare professionals; and (c) government and politics.
1.5 Precancerous Lesion
Leukoplakia is considered as a typical precancerous lesion because some of oral leukoplakia cases become malignant, and some cases diagnosed as leukoplakia have already become cancerous. Malignant transformation rate of oral leukoplakia is discussed in this section.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree