Chapter Outline
GENETIC FACTORS AND FAMILIAL AGGREGATION
Concordance of Childhood Leukemia in Twins
Leukemia and Cancer in the Families of Children with Leukemia
Cancer in Offspring of Patients Treated for Childhood Leukemia
Other Conditions in Relatives of Patients with Leukemia
Human Leukocyte Antigen–DR and Susceptibility to Childhood ALL
PRENATAL ORIGIN OF CHILDHOOD ALL
The Kinlen Hypothesis: Residence in Areas with High Population Mixing
Infections during the Index Pregnancy
Antibiotic Use and Immunizations during the Index Pregnancy
Association of Childhood Leukemia with Factors Related to the Immune System
Early Child Care and Preschool Experiences
Infection and Related Exposures during the Lifetime of the Index Child
Index Child’s Contact with Animals
Leukemia and Bacillus Calmette-Guérin Vaccination
Maternal Diet and Vitamin Supplement Use during Pregnancy
Postnatal Diet of the Index Child and Use of Vitamin Supplements
MEDICAL HISTORY OF THE INDEX CHILD
Leukemias of childhood are a highly heterogeneous group of diseases. In reviewing the descriptive and analytic epidemiology of these malignancies, we have, when possible, emphasized specific subgroups as defined by morphologic, cytogenetic, or molecular features. In selected instances, evidence indicates that specific subgroups of leukemia may have distinct causes and that molecular abnormalities associated with particular subgroups may be linked with specific causal mechanisms. In assessing risk factors, studies of the childhood leukemias present several methodologic advantages compared with those addressing adult leukemias. For example, because the interval between exposure to putative risk factors and the onset of leukemia is shorter, recall of exposures is likely to be more accurate and intervening factors are less likely to be of importance than are those associated with adult leukemias. These characteristics of childhood leukemia better lend themselves to an approach that includes both population studies and molecular epidemiologic techniques, permitting the design of studies to assess interactions between genes and the environment. However, in striking contrast to the impressive advances in the treatment and biology of childhood leukemia, remarkably little has been achieved regarding our understanding of the cause of leukemia, the most common form of childhood malignancy.
Descriptive Epidemiology
Leukemias are the most common cancers affecting children, accounting for 32% of all occurrences of cancer among children younger than 15 years and 27% of occurrences of cancer among children younger than 20 years. Each year in the United States, leukemia is diagnosed in approximately 3540 children who are younger than 20 years. Of these occurrences, acute lymphoblastic leukemia (ALL) accounts for 73%, acute myeloid leukemia (AML) accounts for approximately 18%, and chronic myeloid leukemia (CML) is rarely seen, accounting for less than 4%.
International Patterns
A wide variation exists in the incidence of childhood leukemia by geographic location. The highest annual incidence rates are reported in Costa Rica, Ecuador, Hong Kong, Denmark, and Singapore (57.9 to 51.0 per million population), whereas some of the lowest rates are found in Zimbabwe, India, Israel, and Algeria (23.1 to 26.0 per million). The annual incidence of childhood leukemia for many regions, including North America, Australia, Northern and Western Europe, China, Japan, the Philippines, and Singapore, ranges between 35 and 50 per million ( Table 40-1 ). When evaluating geographic variation in disease incidence, concerns always exist regarding the quality and completeness of reporting. Ecologic studies of childhood leukemia incidence according to annual per-capita gross national income demonstrates substantial variation within low-income countries and a much narrower range in middle- and high-income countries. In addition, a significant correlation exists between the incidence of childhood leukemia and population mortality rates for children younger than 5 years in low- and middle-income countries. Some of the lowest reported childhood leukemia rates are within countries with high mortality rates for children younger than 5 years, suggesting that children with undiagnosed leukemia may die in some low-income countries as a result of anemia or fever that is attributed to infectious disease.
| Country | ANNUAL INCIDENCE RATE (PER MILLION POPULATION) * | ||
|---|---|---|---|
| Leukemia | ALL | AML | |
| Costa Rica | 57.9 | 46.3 | 8.9 |
| Ecuador | 56.3 | 39.6 | 9.1 |
| Hong Kong | 53.9 | 40.6 | 3.9 |
| Denmark | 53.0 | 42.8 | 8.4 |
| Singapore | 51.0 | 39.5 | 6.9 |
| Canada | 50.8 | 41.0 | 6.3 |
| Finland | 49.9 | 41.9 | 5.4 |
| Australia | 49.9 | 39.9 | 8.0 |
| Italy | 49.1 | 37.9 | 7.9 |
| Sweden | 48.7 | 40.1 | 6.7 |
| Norway | 48.6 | 38.3 | 8.0 |
| Philippines | 48.1 | 25.2 | 8.6 |
| United States (white) | 46.9 | 38.0 | 6.0 |
| Germany | 46.6 | 39.0 | 6.7 |
| Uruguay | 44.0 | 29.3 | 7.4 |
| Colombia | 42.8 | 31.5 | 6.4 |
| United Kingdom | 40.8 | 32.8 | 6.3 |
| France | 40.5 | 31.7 | 6.1 |
| China | 40.3 | 17.4 | 6.7 |
| Netherlands | 40.1 | 30.9 | 5.8 |
| Japan | 38.5 | 22.6 | 7.2 |
| Korea | 38.1 | 20.2 | 8.7 |
| Cuba | 37.7 | 25.4 | 5.7 |
| Czech Republic | 37.1 | 28.4 | 5.1 |
| India (Delhi) | 36.0 | 23.1 | 6.1 |
| Peru | 35.9 | 25.4 | 6.9 |
| Brazil | 33.7 | 21.9 | 5.3 |
| Thailand | 29.8 | 19.8 | 5.8 |
| United States (black) | 29.4 | 20.8 | 6.2 |
| Algeria | 26.0 | 14.3 | 5.7 |
| Israel | 25.7 | 18.6 | 5.3 |
| India (Bombay) | 25.4 | 16.0 | 4.8 |
| Zimbabwe | 23.1 | 11.6 | 11.0 |
Age-Specific Incidence and Sex Ratio
The incidence rate for all leukemias is highest among children younger than 5 years and decreases with age. The age-adjusted incidence for boys exceeds that for girls, with a sex ratio typically between 1.1 : 1 and 1.4 : 1.
Acute Lymphoblastic Leukemia
In developed countries, the age-incidence curve for ALL is characterized by a peak between the ages of 1 and 4 years ( Fig. 40-1 ). Thus a sharp peak in ALL incidence is seen among 2- to 3-year-olds (>100 per million), which decreases to a rate of 20 per million for 8- to 10-year-olds. The incidence of ALL among 2- to 3-year-olds is approximately fourfold greater than that for infants and is nearly tenfold greater than that for 19-year-olds. Although this age distribution is well recognized and attributable to common ALL (cALL), it has not always been present. It was first observed in England and Wales in the 1920s, among American whites in the 1940s, among African Americans and in Japan in the 1960s, and among Jews and non-Jews in Israel in the 1970s. In Kuwait, where the incidence was low during the 1970s, the age peak has recently been observed, whereas in the African series, in which the incidence is also low, the peak has not yet been reported.
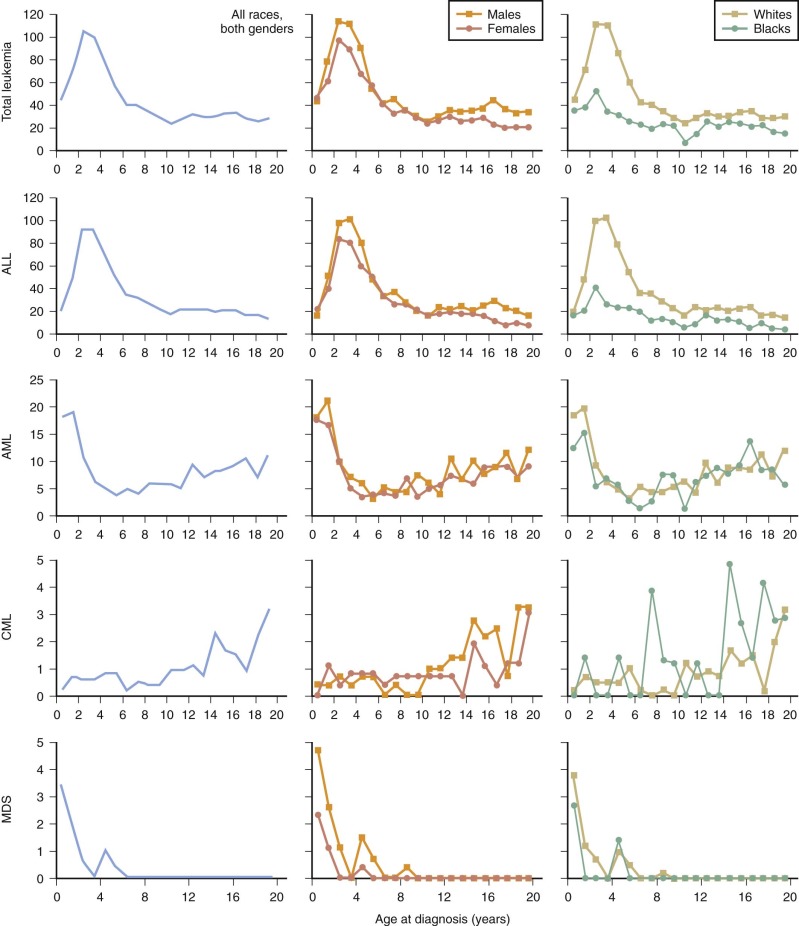
An exaggerated peak incidence among 2- to 3-year-olds, with a very low incidence of ALL in older children, was observed in certain areas of England and Wales and in the rural north of Scotland and coincided with a high socioeconomic status. These observations have led to a hypothesis that an elevated risk of ALL is influenced by community characteristics such as isolation, high socioeconomic status, and population mixing, which in turn are related to immunologic isolation in infancy and influence patterns of exposure to common infectious agents in the early years of life before the appearance of leukemia. These hypotheses are discussed in detail later. The ratio of incidence rates of ALL for boys and girls ranges between 1.1 : 1 and 1.3 : 1.
Acute Myeloid Leukemia
Among children, the incidence rates for AML are highest in infancy and are fairly uniform in older children (see Fig. 40-1 ). In the United States, during 1986 to 1995, the incidence in the 0- to 4-year-old age group was 10.3 per million, and in the 5- to 9-year-old and 10- to 14-year-old groups it was 5.0 per million and 6.2 per million, respectively. Comparable rates have been reported elsewhere in Europe and in England. The male-to-female ratio is close to unity for AML.
Chronic Myeloid Leukemia
The incidence rates of CML in one of the largest series, originating from the United Kingdom, were 1.2 per million for infants and 1.6, 0.5, and 0.7 for children aged 1 to 4 years, 5 to 9 years, and 10 to 14 years, respectively, which are comparable to rates observed in the United States for these age ranges. The rate for CML increases during the 15- to 19-year age interval. The male-to-female sex ratio is 1.6 : 1.
Myelodysplastic Syndrome and Other Myeloproliferative Diseases
The International Classification of Childhood Cancer includes a classification for myelodysplastic syndrome (MDS) and other myeloproliferative diseases. In the 0- to 19-year age group, rates for MDS are very similar to those for CML; however, in contrast, the incidence of MDS is highest among infants and drops essentially to 0 by age 8 years. The incidence is almost twice as high among males.
Ethnic Origin
In the United States, substantial variation is seen in the incidence rates of ALL by ethnicity. The highest rates are reported among Hispanics, Filipinos, and Chinese, and the lowest rates are reported among African Americans. The incidence rates among whites are moderate to high by international standards, whereas those for American Indians are somewhat lower.
On the other hand, two studies have investigated the incidence of childhood cancer by ethnicity in England, but results were negative. The lack of variation in the incidence of ALL among ethnic groups in England, in conjunction with the markedly lower incidence of ALL in the Indian subcontinent and Africa than in England, suggests that the incidence of ALL depends to some extent on environmental factors in association with geographic location. The contrast between the ethnic variations in incidence could be a reflection of the socioeconomic differences by ethnicity (or the lack thereof) in the two countries. A recent report from the Malaysia-Singapore Leukemia Study Group describes differences in prognostically important chromosomal abnormalities among an unselected multiethnic Asian population. Ethnic groups differed significantly with regard to the proportion of patients with ALL who had T-cell ALL (the incidence is higher in Chinese and Indians), ETV6-RUNX1 (with a lower incidence in Chinese persons), and BCR-ABL (with a lower incidence in Indian persons). Beyond ethnic differences in the occurrence of childhood ALL, it has been well documented that race/ethnicity is associated with prognosis. The black-to-white ratio of the incidence of AML in the United States is 0.85. The rates are highest among Hispanics. This higher rate of AML contributed to the higher incidence of acute promyelocytic leukemia seen among Hispanics than among non-Hispanics, thus raising the question of genetic predisposition to acute promyelocytic leukemia or exposure to distinct environmental factors. As with ALL, race/ethnicity also has prognostic importance.
Socioeconomic Status
Socioeconomic factors have been proposed as an explanation for the age peak in childhood leukemia. Thus it has been hypothesized that with economic development, poorer communities move from a situation in which leukemia is rare (T-cell ALL is the predominant type of ALL) through an intermediate stage in which cALL begins to appear to a state of high socioeconomic status that is associated with a high incidence of ALL and cALL. Numerous descriptive studies have investigated the relationship between leukemia and socioeconomic status. In the vast majority of these studies, the area of residence of the patients (or those who died) was used as a measure of socioeconomic status. Other measures used included household income and years of schooling. In most of these studies, a weak positive association between leukemia and high socioeconomic status was observed, with a few exceptions. In contrast, several investigators have examined the relationship between AML and socioeconomic status and have failed to show an association.
Time Trends
Several investigators have examined temporal trends for leukemia, but the findings are difficult to interpret, primarily because of the varying periods covered and different methods of analysis used. Some investigators analyzed the 0- to 14-year-old age group as a whole, whereas others examined trends in specific age groups. Some investigators reported trends for each sex, whereas others combined the two sexes in their analyses. In some analyses all leukemias were grouped together, whereas others conducted their analyses by the leukemia subtype. In addition, diagnostic improvements have led to more accurate and precise classification of leukemias over time, which could contribute to the observed temporal trends in incidence.
In the studies reporting an increase in the incidence of ALL, the increase is fairly modest and is confined to the 0- to 4-year-old age group. Moreover, in some reports, the increase in incidence of ALL appeared to be accompanied by a decrease in the incidence of AML or other leukemias, which could reflect diagnostic shifts and more accurate and precise classification. A temporal increase in the incidence of childhood ALL has been reported in England and is attributed to an increase in the precursor B-cell subtype. Between 1974 and 2000, the average annual increase in childhood ALL was 0.7% overall but 1.4% for cALL. Similarly, analyses of childhood ALL time trends in Italy demonstrated increases, some of which were related to maternal time variables that included maternal age and maternal birth cohort, which were considered to be consistent with an infectious cause. On the other hand, in another study, Linet and associates report no substantial change in the incidence of childhood leukemia diagnosed in the United States between 1975 and 1995. The Czech national registries reported a 1.5-fold increase in childhood ALL incidence between the ages of 1 to 4 years during the period of substantial socioeconomic transition in the European postcommunist countries.
Clustering of Leukemia in Space and Time
Spatial clustering of childhood leukemia has been observed to varying degrees in England, France, and Greece but not in the United States. Where spacial clustering was identified, it was strongest for leukemias diagnosed in younger children. Although spatial clustering was confined to sparsely populated areas in England, such clustering was found only in urban areas in Greece. Birch and colleagues addressed the issue of space-time clustering by using the Manchester Children’s Tumor Registry. All methods showed highly significant evidence of space-time clustering on the basis of place of birth and time of diagnosis of childhood leukemia. The authors concluded that the results were consistent with an infection hypothesis (discussed later). Furthermore, the investigators found an excess of instances in males versus females in space-time pairs, thus suggesting gender-specific susceptibility to infections. More recently they have reported statistically significant cross-clustering among childhood leukemia and central nervous system (CNS) tumors and between ALL and astrocytoma, suggesting possible common causes, possibly of an infectious nature. The correlation between childhood leukemias and CNS tumors is of interest, given the recently reported correlation in international rates for these two pediatric cancers.
Historically, clusters of childhood leukemia have been observed in which the number of cases reported exceed those expected within a given time and geographic location. Investigation of these clusters has typically not identified any causal agent. A recent leukemia cluster in the United States gained substantial public and scientific attention because of the marked excess observed within a small population. Comprehensive investigation including biologic samples from persons within the population that were analyzed for chemicals, viral markers, and genetic polymorphisms, in conjunction with air, water, soil, and dust sampling, failed to identify any leukemia risk factor.
Genetic Factors and Familial Aggregation
Certain genetic syndromes are associated with an increased susceptibility to leukemia. These syndromes include Down syndrome, neurofibromatosis type 1, and chromosome breakage syndromes, such as ataxia telangiecatsia, Bloom syndrome, Shwachman syndrome, Fanconi anemia, and Langerhans cell histiocytosis. Shaw and associates reported an association between Klinefelter syndrome and childhood leukemia on the basis of an observation of two children with ALL who had a 47XXY karyotype. However, Hasle and colleagues subsequently reported no increase in the incidence of childhood leukemia in a cohort of 696 men with Klinefelter syndrome. In a recent study of malformation syndromes among children with cancer, an association was reported between Bardet-Biedl syndrome and ALL.
Although specific associations have been described mainly as case reports, data on the proportion of instances of leukemia that are known to have a genetic etiology or are associated with specific genetic syndromes are limited. In a study from England, 2.6% of children with leukemia were reported to have a recognized genetic condition, and this percentage was almost entirely accounted for by Down syndrome (2.3% of all leukemias). This proportion is similar to that found in studies from the United States and Nordic countries.
Population-based studies using record-linkage approaches have documented that approximately 97% of cancers occurring among children with Down syndrome are leukemia. Persons with Down syndrome have an estimated 56-fold increase in leukemia between the ages of 0 to 4 years, which declines to tenfold for leukemia risk between the ages of 5 to 29 years. More recently, interest has been increasing in the identification of environmental factors that may contribute to the development of leukemia among patients with Down syndrome. Case-control investigations comparing persons with Down syndrome who have leukemia with persons with Down syndrome who do not have leukemia have found that reduced risk of leukemia is associated with maternal vitamin supplementation in the periconceptional period and reported history of infection in the first 2 years of life.
Concordance of Childhood Leukemia in Twins
Assessment of concordance for childhood leukemia in twins may provide etiologic clues. If childhood leukemia were to have a predominantly genetic cause, monozygotic twins would be expected to be concordant for leukemia more often than dizygotic twins because of their genetic similarity. If childhood leukemia were caused by an abnormal intrauterine environment, it would be expected that if one member of a dizygotic twin pair were affected, the other twin would be affected more often than a nontwin sibling because of the shared environment. Recently, in a study of leukemia in a pooled series from the United States, Canada, and England, only three concordant pairs (1.5%) were found among 197 pairs in which one or both twins had leukemia, with the concordance rate for monozygotic twins reported to be 3.9% (95% confidence interval [CI], 0.8 to 11.1). Although the concordance rate in twins of like sex (probably monozygotic twins) is higher than the zero concordance rate reported for twins of unlike sex (dizygotic twins), the concordance rate in twins of like sex is quite variable. These findings suggest that inherited genetic factors most likely play a relatively small part in the cause of childhood leukemia.
The occurrence of concordant leukemic pairs has been hypothesized to be due to parallel expansions of clones descended from a single, ancestral cell transformed in utero, with a malignant clone arising in one monozygotic twin and entering the circulation of the co-twin through anatomizing placental vessels. This mechanism would account for the early and similar age of onset of leukemia and the similar cytogenetic findings. Support for this hypothesis is provided by the recent observation of shared clonal but nonconstitutional mixed lineage leukemia gene ( MLL) rearrangements in the leukemic cells of three pairs of monozygotic twins concordant for infant acute leukemia. The rearrangements were not observed in nonleukemic cells of the twins and were not present in the maternal or paternal blood of two of the three twins.
Leukemia and Cancer in the Families of Children with Leukemia
In studies of leukemias of all types combined, no excess of cancer was observed among siblings, parents, or offspring. In studies focusing on acute leukemia, an excess of acute leukemia was observed among siblings, in whom the expected rates were extremely low. Limitations of these studies include the inclusion of distant relatives, in whom verification of cancer becomes problematic, incomplete follow up, and, finally, the inclusion of families with a history of consanguinity.
Cancer in Offspring of Patients Treated for Childhood Leukemia
Few studies have reported the rate of occurrence of cancer in the offspring of patients treated for childhood leukemia. Only recently have enough patients survived that the number of their offspring permitted estimates of their risk of developing malignant neoplasms. After performing a review of the literature, Hawkins and associates estimated that the proportion of heritable cases among survivors is unlikely to exceed 5%, assuming that the age of onset of all heritable cases is 15 years of age or younger and that the diseases had a penetrance of 70% or more. Chromosomal instability was examined in 20 apparently healthy children of survivors of childhood malignancy. Compared with control subjects, no increases in spontaneous or bleomycin-induced aberrations were found in these “index children.” The results suggest that the offspring of subjects who previously received chemotherapy, radiotherapy, or both for childhood malignancy probably have no increased risk of latent chromosomal instability.
Other Conditions in Relatives of Patients with Leukemia
Savitz and Ananth reported an excess of major birth defects among the siblings of patients with ALL (relative risk [RR], 3.2; 95% CI, 1.3 to 7.7; adjusted for age at diagnosis, sex, and year of diagnosis). Mann and colleagues reported an excess of congenital defects among parents, uncles, aunts, and other distant relatives of patients with ALL compared with community control subjects. Buckley and associates reported an excess of a number of conditions in siblings, parents, and grandparents of index patients with various types of ALL. These conditions included musculoskeletal disorders among relatives of patients with the common cell type; gastrointestinal, hematologic, and musculoskeletal disorders and allergies for those with the pre–B-cell type; an excess of gastrointestinal disorders for those with the T-cell type; and an excess of congenital heart and lung disease for those with the null cell type. The offspring of adult survivors of childhood ALL were not found to have an increased risk of occurrence of congenital anomalies compared with the offspring of sibling control subjects. Similarly, Kenney and colleagues reported no excess of congenital anomalies among the offspring of adult survivors of childhood cancer compared with the offspring of sibling control subjects.
Human Leukocyte Antigen–DR and Susceptibility to Childhood ALL
Both genetic and environmental factors could possibly play an interactive role in the development of childhood ALL. Because of the demonstration of the influence of major histocompatibility complex on mouse leukemia, a human leukocyte antigen (HLA) association has been considered as a possible genetic risk factor. Dorak and associates demonstrated a moderate association with the most common allele in the HLA-DR53 group, HLA-DRB1*04, which was stronger in males. In addition, homozygosity for HLA-DRB4*01, encoding the HLA-DR53 specificity, was increased among patients. Confounding these associations was a male-specific increase in homozygosity for HLADRB4*01. The cross-reactivity between HLA-DR53 and H-2Ek, extensive mimicry of the immunodominant epitope of HLA-DR53 by several carcinogenic viruses, and the extra amount of deoxyribonucleic acid (DNA) in the vicinity of the HLA-DRB4 gene provide arguments for the case that HLA-DRB4*01 may be one of the genetic risk factors for childhood ALL. Comparison of DQA1 and DQB1 alleles in 60 children with ALL and 78 newborn infants (control subjects) revealed that male but not female patients had a higher rate of occurrence of DQA1 *0101/*0104 and DQB1 *0501 than did appropriate control subjects. The authors concluded that this finding represented a male-associated susceptibility haplotype in ALL, supporting an infectious cause. Additional investigations from the United Kingdom have suggested that cALL in children may be associated with HLA-DPB1 through a mechanism that involves the presentation of specific antigenic peptides, possibly derived from infectious agents, which leads to activation of helper T cells that mediate proliferative stress on preleukemic cells.
Susceptibility to Childhood ALL: Genetic Polymorphisms
In the search for gene-environment interactions in the cause of childhood leukemia, investigations have focused on the genetic variability in xenobiotic metabolism, DNA repair pathways, and cell-cycle checkpoint functions that might interact with environmental factors to influence leukemia risk. Although much of the research in this area has been limited by small sample size, data suggest a potential role for polymorphisms in genes encoding cytochrome P450, glutathione S-transferase, reduced nicotinamide adenine dinucleotide phosphate quinone oxidoreductase, methylenetetrahydrofolate reductase (MTHFR), cycle-cycle inhibitors, and DNA-repair polymorphisms.
In general, few reports have addressed the potential role of these polymorphic genes in childhood leukemia, and in many instances they provide contradictory findings. This situation is particularly true with regard to studies of GSTM1 and GSTT1 and CYP-P450 enzymes. Low folate intake or alterations in folate metabolism as a result of polymorphisms in the enzyme MTHFR have been associated with neural tube defects and some cancers. Polymorphic variants of MTHFR lead to enhanced thymidine pools and better quality DNA synthesis that could afford some protection from the development of leukemias, particularly those with translocations. Wiemels and colleagues and Smith and colleagues reported an association of MTHFR polymorphisms in infant leukemias with MLL gene rearrangements and childhood leukemia with either ETV6-RUNX1 fusions or hyperdiploid karyotypes. These studies provide evidence that molecularly defined subgroups of childhood leukemias may have different causes and also suggest a role for folate in the development of childhood leukemia. In a case-control study, MTHFR genotypes TT677/AA1298 and CC677/CCC1298 were associated with a reduced risk of childhood ALL. Moreover, the findings of these studies suggested a gene-environment interaction based on timing of implementation of folic acid supplementation in the Canadian population. To date, no direct gene-environment associations have been established convincingly. When considering the potential importance of the prenatal origin of childhood leukemia, it becomes important to consider the genotypic profile of the mother with respect to metabolizing enzymes. Investigation of maternally mediated genetic effects through reduced nicotinamide adenine dinucleotide phosphate quinone oxidoreductase did not affect risk for childhood ALL.
Prenatal Origin of Childhood ALL
As mentioned earlier, molecular studies on several pairs of identical twins, aged between 2 months and 14 years at diagnosis, first provided strong evidence that concordant ALL arises in monozygotic twins after mutation and clonal expansion of one cell in one fetus in utero. Because the disease in the twins is not clinically or biologically different from that in singletons, Gale and associates hypothesized that some of the singletons are likely to have a prenatal initiation of their leukemia. They further hypothesized that an additional event or exposure postnatally results in the clinically overt leukemia at a variable time after birth. To test these hypotheses, they used the neonatal blood spots or Guthrie cards to identify the presence of clonotypic or patient-specific leukemia fusion-gene sequences (ETV6-RUNX1) . The association between the t(12;21) and the deletion of the nontranslocated allele of TEL is among the most frequent abnormalities observed in B-lineage ALLs. Neonatal blood samples are routinely used to screen for inherited metabolic disorders. By reverse-transcriptase polymerase chain reaction screening of blood or bone marrow, the investigators identified ETV6-RUNX1 fusion in 12 children and a pair of identical twins, aged 2 to 5 years, with newly diagnosed ALL. They identified ETV6-RUNX1 fusion sequences in blood spots from the identical twins and in six of the nine studied ETV6-RUNX1–positive patients. Gale and colleagues concluded that childhood ALL is often initiated by a chromosome translocation event in utero. This important observation has been more extensively investigated and characterized. Screening of neonatal cord/blood samples has revealed a putative leukemia clone with the ETV6-RUNX1 fusion gene in 1% of newborn babies, which represents a frequency 100 times greater than the prevalence of ALL defined by this fusion gene later in childhood. In children with cALL who have a ETV6-RUNX1 fusion gene, deletion of the unrearranged or normal TEL allele is a common secondary occurrence. The causal events that lead to the initial ETV6-RUNX1 fusion in utero and any postnatal secondary mutations are not known. Some epidemiologic data provide support for the idea that an abnormal immunologic response under particular social conditions could account for certain critical postnatal events. Further epidemiologic studies need to focus on in utero exposures that could result in the initial ETV6-RUNX1 fusion and postnatal exposures or events that could explain the secondary mutations.
Infection
Considerable interest and research has focused on the potential role of infection in the cause of childhood ALL through a mechanism of stimulation of an inappropriate immune response or via a direct transformation. This avenue of research, proposed and spearheaded by British researchers, reflects two different but conceptually related hypothesized mechanisms.
The Kinlen Hypothesis: Residence in Areas with High Population Mixing
In 1988 Kinlen hypothesized that the high incidence of leukemia in children in the vicinity of the Sellafield and Dounreay nuclear reprocessing plants in England could be due to a rare response to some unidentified mild and subclinical infection, the transmission of which is facilitated by contacts between large numbers of people. An influx of people of diverse origin into a previously isolated area would particularly facilitate transmission. Thus a high incidence of leukemia in childhood would be expected in isolated areas into which a significant influx of people had occurred. The hypothesis was first tested in an area in Scotland. Glenrothes New Town was identified a priori as an area where, after a period of relative isolation, a sudden burst of in-migration occurred in 1964 because of the opening of the Forth Road bridge. A significant number of persons younger than 25 years who died of leukemia were identified during the period from 1951 to 1967. The incidence of leukemia was not excessive in the period from 1968 to 1985, when the community became much less isolated. The hypothesis has been tested in a number of other situations in England, France, and other parts of Europe and Hong Kong. * A 3.6-fold increase in childhood leukemia was observed in the wartime cohort compared with national Scottish rates in Orkney and Shetland (England’s northernmost islands) during World War II, when the local people were outnumbered by servicemen stationed there. The rates normalized to the national rates in the postwar period. A recent analysis of the U.S. Surveillance Epidemiology and End Results data also provided confirmatory evidence for the population mixing hypothesis, with findings that changes in rural county population sizes from 1980 to 1989 were associated with increased incidence rates for childhood ALL.
* References .
Infections during the Index Pregnancy
An alternative model for the cause of childhood ALL places the critical infectious event during pregnancy rather than early childhood. In this model, the etiologic agent causing the primary infection in the mother is transmitted to the fetus, and as a consequence of this in utero infection, the child is at increased risk for the development of ALL before the age of 5 years. The characteristics that the causative infectious agent should possess include: (1) the ability to induce genomic instability, (2) specific effects on B lymphocytes and not on T lymphocytes, (3) higher rates of infection in regions with lower socioeconomic status, (4) limited general oncogenic potential, (5) minimal symptoms associated with the primary infection, and (6) the ability to cross the placenta and infect the fetus but not result in severe fetal abnormalities. A virus that meets several of these criteria is the JC virus, a member of the polyomavirus family.
The possibility that maternal infection during pregnancy could potentially be leukemogenic is supported by the observation that leukemia in cats is caused by a feline virus transmitted from the mother to the fetus. Three cohort studies of maternal infection during pregnancy and subsequent childhood cancer revealed an excess of cancer in these children compared with the age- and sex-matched general population. In case-control studies, no association between maternal infection with influenza or varicella and subsequent childhood cancer was seen. With regard to maternal infection in general, a twofold excess of childhood cancer has been reported. However, the studies are small or the reported prevalence of exposure is low and may be attributed to recall bias. Neonatal blood spots have been used to identify evidence of selected in utero viral infections. Investigation of cytomegalovirus and human parvovirus B19 infection failed to identify DNA in the small groups of childhood ALL cases compared with healthy control subjects. First-trimester serum samples from mothers of children diagnosed with ALL derived from large maternal cohorts in Finland and Iceland have been tested for antibodies to Chlamydia trachomatis , Helicobacter pylori, and Mycoplasma pneumonae. Comparison of ALL cases to control subjects identified an increased risk associated with M. pneumoniae immunoglobulin M and, in Iceland, H. pylori immunoglobulin G. Within the same maternal cohort, evidence was found for activation of maternal Epstein-Barr virus infection in both childhood ALL and non-AML cases.
Smith and colleagues sought to assess the relationship of childhood ALL with hygiene conditions, an aspect of socioeconomic development affecting rates of exposure to infectious agents. The data suggested that improved public hygiene conditions measured by decreased prevalence of hepatitis A virus infection (an agent with a fecal-oral route of transmission) were associated with high childhood ALL incidence rates. The model presented in this study supports the plausibility of the hypothesis that decreased childhood exposure to a leukemia-inducing agent associated with hygiene conditions leads to higher rates of ALL in children by increasing the incidence of in utero transmission caused by primary infection during pregnancy (or by increasing the number of persons infected in early infancy because of a lack of protective antibodies).
Antibiotic Use and Immunizations during the Index Pregnancy
No association between antibiotic use during pregnancy and leukemia has been identified. No consistent association has been reported between vaccination during pregnancy and leukemia. Salonen and Saxen found a positive association between leukemia and maternal polio vaccination during pregnancy. Similarly, Gilman and associates reported a positive association between total childhood deaths and vaccination during pregnancy that was largely accounted for by neoplasms of the reticuloendothelial system. However, results of a population-based study in the Netherlands showed no association between vaccination during pregnancy and ALL.
Association of Childhood Leukemia with Factors Related to the Immune System
It has been proposed that some instances of pediatric ALL arise as a rare response to a common childhood infection and that the leukemia-inducing potential of the agent is related to the timing of infection, with a greater leukemogenic effect for later infections compared with those occurring during infancy. In this context, factors related to the child’s immune system are of special interest. Several investigators have sought to identify factors such as birth order and breastfeeding that can potentially be used as markers of infections or confer protection against infection before the diagnosis of leukemia.
Breastfeeding
Breastfeeding is well known to have a protective effect against infection in infants. Data regarding the association between breastfeeding and subsequent childhood leukemia are important because of the proposed hypothesis about the etiologic role of infections, taking into consideration the fact that breastfeeding protects against infections. This association was examined in several studies with mixed results. Whereas some researchers failed to show any association between breastfeeding and childhood leukemia, other researchers do show that a protective effect is offered by breastfeeding. A report of a large North American case-control study revealed that breastfeeding was associated with a reduced risk of childhood acute leukemia. The inverse association was stronger with a longer duration of breastfeeding for patients with ALL and AML. A recent meta-analysis suggested that breastfeeding of any duration was associated with a 9% lower risk of ALL, but little evidence was found of an association for risk of childhood AML.
Immunizations
Previous studies suggested that vaccinations during infancy may reduce the risk of subsequent childhood leukemia. No consistent association between leukemia and immunization has been identified, although two recent studies suggested that early immunization against Haemophilus influenzae type b may reduce the incidence of childhood leukemia. Auvinen and associates compared the vaccination histories of 439 children in whom ALL was diagnosed and 439 community control subjects matched to patients with regard to age, race, and telephone exchange. No association was demonstrated between most infant vaccinations such as oral poliovirus, diphtheria-pertussis-tetanus, and measles-mumps-rubella. However, infants receiving the conjugate H. influenzae type b vaccine had a reduced risk for subsequent childhood ALL. Confirmatory studies are needed.
Sibship Size
Sibship size might be an indirect indicator of exposure to common childhood infections. However, although no consistent association between ALL and sibship size has been identified, authors of a study from Canada reported that when all variables defining family structure are included in a model, having older siblings at the time of diagnosis was a risk factor among children in whom ALL was diagnosed before 4 years of age, whereas having older siblings in the first year of life was a protective factor among children in whom ALL was diagnosed at 4 years or later. More recently, analysis of the Swedish Family-Cancer Database demonstrated that having three or more older siblings is associated with a significantly reduced risk of childhood AML and ALL.
Early Child Care and Preschool Experiences
Day care of the index child and of siblings has been used as a proxy measure of exposure to infections. The findings of case-control studies have been inconsistent regarding association between attendance at day care centers and risk of leukemia. A recent study conducted by the United Kingdom Childhood Cancer Study (UKCCS) investigators was specifically designed to test the early infection hypothesis, including the role of day care. These investigators found that increasing levels of social activity were associated with reductions in the risk of ALL in a dose-response manner.
Infection and Related Exposures during the Lifetime of the Index Child
Studies of an association between childhood cancer and postnatal infection of the index child are problematic because of the difficulty in assessing whether the episodes of infectious disease were a possible cause of the childhood cancer or represented a prediagnostic sign of the disease. Some investigators have attempted to overcome this concern by restricting attention to infection during the first year or first 6 months of life or by considering infections reported only during a specified period before diagnosis in patients and during a reference time in control subjects. Specifically, investigators explored the role of parvovirus B19 with its hematotropic effects and the potential to precipitate varying forms of cytopenia in patients before or after the diagnosis of ALL, but they failed to find an association between parvovirus B19 and childhood ALL. Other epidemiologic studies have provided inconsistent findings for an association between leukemia and recorded or reported infections in early life. In the UKCCS investigation, which utilized general practitioner records for visits during the first year of life, it was found that children with ALL who were diagnosed between the ages of 2 to 5 years had significantly more clinically diagnosed infectious episodes in infancy. Another case-control study from the UKCCS group explored the relationship between childhood leukemia, infant infection, and three markers of infection exposure: birth order, infant day care attendance, and area-based deprivation. The study findings indicated that immune dysregulation among children who are diagnosed with ALL is detectable from an early age. Moreover, recent epidemiologic data indicate that the risk of cALL is increased by higher socioeconomic status, isolation, and other community characteristics suggestive of abnormal patterns of infection during infancy, and population-based incidence patterns have identified small peaks in the incidence of childhood ALL that coincide with years immediately following influenza epidemics.
Chloramphenicol
Chloramphenicol can cause bone marrow suppression and could potentially play an etiologic role in childhood leukemia. Shu and colleagues reported a positive association with the use of chloramphenicol from a study conducted in Shanghai. The risk of leukemia associated with chloramphenicol use increased with an increasing total number of reported days of use. The association was stronger for AML than for ALL. A major limitation of the study was the long interval between diagnosis (1974 to 1986) and interview (1985 to 1986) for the patients, whereas the control subjects were recruited in the 2-year period of 1985 to 1986.
Index Child’s Contact with Animals
Although considerable interest has been generated in a possible role of feline leukemia virus because of similarities between human and feline ALL in clinical findings, laboratory data, and response to treatment, evidence is lacking for a biologic association.
Bovine leukemia virus has also been studied for a possible role in the cause of human leukemia. A case-control study of 131 children with leukemia and 136 regional population control subjects was conducted to investigate this association. However, none of the DNA samples from patients or control subjects hybridized with a bovine leukemia virus DNA probe, providing strong evidence that genomic integration of bovine leukemia virus is not a factor in childhood ALL.
Buckley and associates reported a positive association with exposure of the mother, father, and index child to farm animals, compared with control subjects who had other types of cancer or community control subjects. However, no overall association with cat ownership was identified. A recent large case-control study failed to show any association between pet ownership and childhood acute leukemia.
Leukemia and Bacillus Calmette-Guérin Vaccination
Laboratory evidence that tubercle bacilli of the Calmette-Guérin strain (BCG) can prevent or suppress challenge from leukemia or tumor grafts in laboratory animals led investigators to explore whether BCG vaccination might reduce the incidence of cancer in humans and of leukemia in particular. However, no consistent association between BCG vaccination and the subsequent risk of leukemia was found.
Seasonal Variations in the Onset of Childhood Leukemia
Seasonal variations in onset of disease could provide supportive evidence of an infectious cause. Westerbeek and colleagues demonstrated significant seasonal variation in the date of the first symptom of childhood ALL, with peaks occurring in November. However, a larger study of 15,835 patients with childhood leukemia failed to reveal any evidence of seasonality in either month of birth or month of diagnosis overall or in any subgroups by age, sex, histologic analysis, or immunophenotype. However, this study did find a statistically significant February peak in month of birth for patients born before 1960 and an August peak in month of diagnosis for patients in whom leukemia was diagnosed before 1962. Although these findings may be due to chance, they are also consistent with changes over time in the seasonality of exposure or immunologic response to a relevant infection. Changes in the seasonal variation in the fatality rate of a preleukemic illness, such as pneumonia, could be another explanation. Ross and associates examined data from the Children’s Cancer Group and the Pediatric Oncology Group for seasonal variation patterns. A statistically significant seasonal variation for ALL was found, with a peak in the summer. Biologic mechanisms underlying these seasonal patterns are probably multifactorial and need to be investigated.
Environmental Exposures
Although a large body of literature describes the associations between a variety of environmental exposures and childhood leukemia, insufficient evidence exists for a causal relationship in most exposure studies, with the exception of those for ionizing radiation. Retrospective studies are limited because of the reliance placed upon parental self-report of the exposure in the context of a case-control study design, which can be hampered by recall bias. In addition, the nonspecific nature of the exposure being assessed makes it difficult to explore causal mechanisms involved with the associations. Moreover, a majority of the exposure assessment has focused on paternal exposures, with little emphasis placed on maternal exposures. To more clearly evaluate the importance of these exposures in future investigations, improvement is needed in the following areas: (1) importance given to maternal exposures; (2) sophisticated exposure techniques used; (3) attention paid to the mechanism, timing, and route of exposure; and (4) the provision of evidence that the exposure is actually transferred to the child’s environment.
Radiation Exposure
In utero exposure to radiation has been reported to be associated with a small yet statistically significant increased risk of development of childhood leukemia in numerous case-control studies. † Reported estimated risks generally ranged from 1.1 to 2.0, with most of the risk ratios being equal to or lower than 1.6. It is reasonable to expect that in utero levels of exposure to radiation would have declined substantially over time given the knowledge of health-related risks associated with radiation exposure and the reduced level of radiation required for diagnostic procedures. Studies from Sweden, England, and the United States have demonstrated higher risk estimates among children born in earlier eras (e.g., the 1930s to 1950s) compared with more recent periods. Nonetheless, the potential role of in utero exposure to radiation in childhood leukemia has remained controversial. Much of the debate has focused on the lack of an observed association among offspring of mothers who were exposed while pregnant at the time of the Hiroshima and Nagasaki atomic bombs coupled with concerns regarding the lack of good exposure assessment and the potential for recall bias in case-control studies of the topic. Results from a large case-control study in North America did not identify an overall increased risk associated with in utero pelvimetric diagnostic x-rays or associations within subgroups defined by immunophenotype. It is likely that the reduced rate and dose of exposure in North America has resulted in an inability to detect the small risk that may exist.
† References .
In contrast to the in utero data, postnatal exposure to radiation has not been consistently associated with the risk of childhood leukemia. Regardless, even if leukemia risk is increased, the potential number of instances of leukemia that currently might be attributed to in utero or postnatal exposure to radiation would probably be very small when one considers the modest magnitude of risk and the limited level of exposure. Results of a case-controlled study of 302 cases of infant leukemia suggested that paternal low-level radiation exposure before conception is associated with an increased risk of infant leukemia, although the nature of this association needs to be further evaluated.A number of investigations of environmental exposures of children to sources of radiation from nuclear detonations and power plants were conducted to determine the potential impact on leukemia risk. In studies of children who survived the atomic bombs of Hiroshima and Nagasaki, an increased occurrence of leukemia 5 to 15 years later was seen. Leukemia risk was positively correlated with the dose of radiation among the atomic bomb survivors. Reports relating to follow-up of nuclear accidents at Three Mile Island and early reports from Chernobyl did not provide strong evidence of an increased risk of childhood leukemia. Analyses that included residents in the area surrounding the Three Mile Island facility did not reveal an increase in the ratio of observed to expected incidence of leukemia. Similarly, investigation of specific regions of the former Soviet Union did not reveal increased rates of childhood leukemia within the first 5 years after the Chernobyl accident. Because of the widespread fallout from Chernobyl, a series of investigations were undertaken in affected countries, including Sweden, Finland, England, Scotland, Germany, and Greece. With the exception of higher rates in Scotland and an observed excess of infant leukemia (i.e., in children younger than 1 year) in Greece, results were generally negative for an observed increase after the accident. The most recent investigation of the acute and long-term risks for childhood leukemia resulting from radiation exposure (a median estimated dose of <10 mGy) after the Chernobyl power station accident identified a significant increase in leukemia risk with increasing radiation dose to the bone marrow. However, the potential overestimate of childhood leukemia risk within one region raised serious concerns about conclusions that could be drawn with regard to the level of risk resulting from the Chernobyl accident.
During the 1950s, testing of nuclear weapons took place in Nevada, with nuclear fallout affecting regions of Nevada and Utah. Studies were carried out in Utah to determine the potential impact on childhood leukemia, using geographic region–specific radiation dose estimates to the bone marrow. No significant trend between estimated dose and risk of leukemia mortality was found. However, the risk of death from leukemia was found to be higher in those exposed at the highest dose level (6 to 30 mGy) compared with those in the lowest dose group (0 to 2.9 mGy).
During the past several decades, the potential impact of nuclear facilities, either nuclear energy plants or nuclear reprocessing plants, has generated considerable interest with regard to cancer risk among surrounding residents. Studies addressing childhood leukemia have been conducted in England, France, the United States, Germany, and Canada. In aggregate, these investigations have not provided strong evidence of an increased occurrence of childhood leukemia, although several have reported increases in incidence and mortality.
Although the biologic plausibility of indoor radon exposure and the cause of childhood leukemia are speculative and debatable, studies have been conducted to address the topic. Although ecologic investigations have provided some evidence for a correlation, case-control studies incorporating measurement of indoor radon levels have not found higher levels within homes of children with ALL or AML.
Parental Occupational Exposures
Occupational exposure of parents and its association with childhood leukemia have been the subject of several investigations. Occupations and exposures of fathers were investigated more often than were those of the mother. Detailed assessment with use of a job exposure matrix to classify occupational exposures identified an increased risk for childhood AML in association with maternal exposure to 1,1,1-tricholorethane, toluene, mineral spirits, alkanes, and mononuclear aromatic hydrocarbons, although dose-response associations were not present. Exposure in the home was not associated with increased risk. Investigation within the UKCCS identified small but statistically significant increased risks among children with ALL whose fathers reported their occupation to involve motor vehicle driving and were exposed to exhaust fumes and/or inhaled particulate hydrocarbons. The studies have several limitations related to the quality of exposure assessment, small numbers of exposed children, multiple comparisons, and possible bias toward the reporting of positive results. Although some evidence exists for an association between childhood leukemia and paternal exposure to solvents, paint, and employment in motor vehicle–related occupations, clearly established associations between parental occupation and childhood leukemia are lacking.
Exposure to Chemicals and Dust
Pesticides
The association between pesticides—including insecticides, fungicides, and herbicides—and childhood leukemia has generated considerable interest, primarily because of the observed higher incidence of leukemia in rural areas and because of the biologic activity of these chemicals. Leiss and Savitz reported a positive association between the use of pest strips and childhood leukemia. The insecticide used in pest strips was dichlorvos, an organophosphate that has been associated with adult-onset leukemia in men. Authors of another study reported an association between the use of pesticides in gardens and farms and childhood leukemia, but no association of leukemia with reported home pest extermination was found in this study. A positive association between maternal exposure during pregnancy and lactation and paternal exposure to household pesticides and garden pesticides or herbicides during the time the mother was pregnant was reported by Lowengart and associates. A recent study provides further evidence for an association between an increased risk of leukemia and rural residence and use of household pesticides. Authors of several other studies report an association between pesticide exposure and childhood leukemia.
In a multicenter study of 404 patients who had ALL and individually matched community control subjects, Buckley and associates reported a positive association between ALL and both paternal exposure (RR, 2.8; P < .001) and exposure of the index child (RR, 5.0; P < .001) to insecticides. The strongest associations for both paternal exposure and exposure of the index child were apparent for T-cell and common cell ALL.
In another case-control study consisting of 204 patients with AML and matched community control subjects, Buckley and associates reported a positive association with maternal occupational exposure to pesticides before, during, and after pregnancy and paternal occupational exposure during the same period. In addition, an independent association of maternal exposure to household fly sprays, pesticides, and garden and agricultural sprays and treatment of the house by insect exterminators in the month before the last menstrual cycle and during the index pregnancy was found, along with direct exposure of the index child to household and garden insecticides. These associations were independent of parental occupational exposure.
Thus, although a large body of literature exists regarding the association between pesticide exposure and childhood leukemia, the studies are limited by the nonspecific nature of the exposure, the reliance of these studies on parental self-report, and the lack of sufficient evidence for a causal relationship. Moreover, use of pesticides may be an indicator of rural isolation, and thus there is a possible confounding effect because of the patterns of exposure to infection that may be associated with population mixing.
Agent Orange.
Wen and colleagues reported a small increase in the risk of the diagnosis of AML before the age 2 years among offspring of veterans who had served in Cambodia or Vietnam. The etiologic importance of these observations remains to be determined. Exposure to Agent Orange was evaluated in this study, but no association was identified. Other studies also concluded that exposure to the herbicides in Agent Orange was not associated with an increased risk of childhood leukemia among the children born to veterans who reported the exposure.
Hydrocarbons and Solvents
Parental exposure to hydrocarbons at work has been investigated as a potential risk factor for childhood leukemia, with no consistent association between paternal or maternal occupational exposure to hydrocarbons and leukemia (all types and specifically ALL). Similarly, no association between paternal exposure to benzene and leukemia has been found. Maternal occupational exposure has been less well studied, but in two of the three studies, a positive association with maternal occupational exposure to hydrocarbons was found that is compatible with the observation that benzene is associated with AML in adults. Two studies indicated that maternal exposures to hydrocarbons during the preconception period, during pregnancy, and during the postnatal period were related to an increased risk of ALL. A positive association between ALL and paternal exposure to hydrocarbons during the preconception period was also found.
No clear association with residential proximity to industrial sources of hydrocarbons has been observed, although authors of one study reported an increased risk of childhood leukemia, and AML in particular, associated with living near petrol stations and/or repair garages. An increased risk of childhood leukemia associated with paternal occupational exposure to chlorinated solvents was reported by several investigators. The risk has been assessed for exposure during the periods of 1 year before pregnancy, during pregnancy, and after delivery. Several investigators reported a positive association with postnatal exposure of the index child to solvents. No consistent association with maternal exposure to solvents is apparent. A recent study revealed a significant association between substantial participation by household members in some common household activities involving organic solvents and childhood leukemia, but further substantiation of these findings is necessary.
Metal Dusts and Fumes
A positive association between the total reported duration of paternal occupational exposure to lead and AML has been reported by Buckley and associates. In the same study, these investigators reported an association between maternal occupational exposure to “metal dusts and fumes.” Similar findings were reported by Shu and colleagues ; in their study in Shanghai, maternal occupational exposure to lead was associated with an increased risk of acute leukemia. A positive association between maternal exposure to molten metal and ALL (null cell and T cell) and a positive association between paternal exposure to metals and T-cell ALL have been reported. However, ecologic studies have failed to show an association between leukemia and proximity to industrial facilities with an increased exposure to metal or metal fumes.
Wood Dust
An association between maternal occupational exposure to wood dust before conception of the index child and childhood leukemia and non-Hodgkin lymphoma has been reported. A few women were exposed during or after pregnancy. Significantly elevated RRs have been reported for paternal occupational exposure to wood dust before conception, during the periconceptional and gestational periods, and postnatally. The association of exposure to wood dust and leukemia was studied by Buckley and associates. They found that the risk was elevated compared with that for other cancers and also compared with that for community control subjects.
Traffic
Occupational exposure to elevated concentrations of benzene is a known cause of leukemia in adults. Concentrations of benzene from motor vehicle exhaust fumes could be elevated along highly trafficked streets. The hypothesis that exposure to traffic-related air pollution increases the risk of development of childhood cancer was examined by several investigators. Results indicate that an association might exist between residence on a busy street and childhood leukemia. However, these results need to be viewed with caution, because most of the results are based on ecologic studies, with an imperfect assessment of individual exposure.
Electromagnetic and Radiofrequency Field Exposure
An initial report in 1979 suggested an association between childhood leukemia and residential proximity to sources of electric and magnetic fields, as assessed by wiring configurations. Subsequently, a large number of investigations have been conducted to further evaluate the hypothesis that exposure to extremely low-level electric and magnetic fields may influence the risk of childhood leukemia and other pediatric malignancies. ‡ These studies, although they provide some consistent findings, are difficult to interpret given the inconsistencies observed in associations between inferred exposure (i.e., wire configuration) compared with measured fields.
‡ References .
Moreover, many of these studies are limited by small sample size and the inability to adequately consider potential confounding factors within the analysis. Three of the more recently reported investigations from the United States, Canada, England, and Japan provide rather convincing evidence that electric and magnetic field exposure is not associated with a significantly increased risk of childhood ALL. Nonetheless, results of meta-analyses have been interpreted to suggest that risk may be increased at the highest exposure levels (i.e., >0.4 µT). However, it is important to note that even if an increased risk does exist at this highest exposure level, the proportion of the population exposed is extremely small and thus the attributable risk would be negligible. Beyond investigation of risk for leukemia, reports have been made of possible associations between electric and magnetic field exposure and prognosis.Although not as extensively studied as electric and magnetic fields, radiofrequency exposure has been suggested as a possible cancer-related risk factor. A recent report investigating radiofrequency exposure in 1928 patients with childhood leukemia observed a twofold risk associated with living within 2 km of an amplitude modulation radio transmitter, which was statistically significant in a dose-dependent fashion for childhood ALL.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree