Fig. 1.1
Age-adjusted Surveillance, Epidemiology, and End Results (SEER) incidence rates by age at diagnosis/death; breast (in situ), female, all races, female 1975–2011 (SEER 9; Cancer sites include invasive cases only unless otherwise noted. Rates are per 100,000 and are age-adjusted to the 2000 US Std Population (19 age groups—Census P25—1130). Regression lines are calculated using the Joinpoint Regression Program Version 4.1.0, April 2014, National Cancer Institute. Incidence source: SEER 9 areas (San Francisco. Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta))
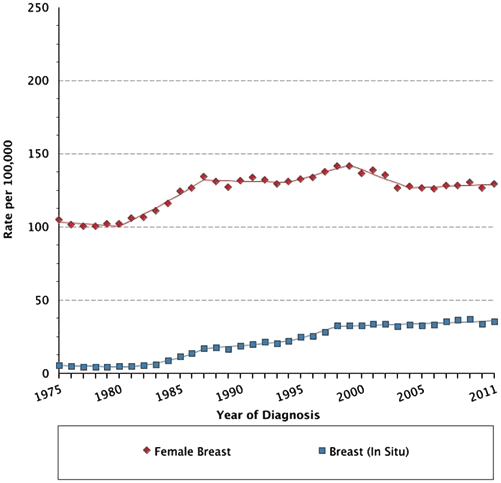
Fig. 1.2
Age-adjusted Surveillance, Epidemiology, and End Results (SEER) incidence rates by cancer site, all ages, all races, female 1975–2011 (SEER 9; Cancer sites include invasive cases only unless otherwise noted. Rates are per 100,000 and are age-adjusted to the 2000 US Std Population (19 age groups—Census P25—1130). Regression lines are calculated using the Joinpoint Regression Program Version 4.1.0, April 2014, National Cancer Institute.)
International Comparisons
As breast-screening programs have been implemented in many countries around the world, the rates of DCIS have similarly increased. In a study of women aged 50–69 years from 15 screening programs across 12 International Cancer Screening Network (ICSN) countries between 2004 and 2008, the overall incidence of DCIS averaged 16 % (0.82 per 1000 examinations) with incidence being the highest in the USA (24 %; 95 % confidence interval (CI): 22–25 %) and the lowest in Finland (9 %; 95 % CI: 8–10 %; Table 1.1 [10]. Sorum et al., using data from the Norway Cancer Registry and the Norwegian Breast Cancer Screening Program (NBCSP) , found that the incidence of DCIS increased from 4 per 100,000 women-years before implementation of the screening program (1993–1994) to 11 per 100,000 women-years after implementation (2006–2007). The proportion of cases in whom DCIS was found was higher among screen-detected cases than the non-screen-detected cases (18 % vs. 5.5 %), after NBCSP was fully implemented [11].
Table 1.1
Total number of tests, DCIS cases, and age-standardized DCIS detection rates per 1000 women aged 50–69 years from 15 screening programs across 12 International Cancer Screening Network (ICSN) countries: 2004–2008 [10]
Country/Region | Total tests | DCIS cases (n) | DCIS cases percent (%) | DCIS cases per 1000 tests | DCIS per 1000 subsequent tests |
|---|---|---|---|---|---|
Czech Republic | 699,726 | 359 | 10 | 0.51 | – |
Denmark/Copenhagen | 47,249 | 73 | 19 | 1.55 | 1.38 |
Denmark Fyn | 97,176 | 63 | 10 | 0.64 | 0.62 |
Finland | 862,908 | 361 | 9 | 0.45 | 0.44 |
Ireland | 331,854 | 393 | 19 | 1.21 | 1.01 |
Italy | 1,453,292 | 1066 | 15 | 0.72 | – |
Japan | 106,898 | 72 | 23 | 0.66 | 0.62 |
Luxembourg | 45,586 | 48 | 16 | 1.06 | 1.06 |
Netherlands | 718,202 | 576 | 16 | 0.80 | 0.76 |
Norway | 963,424 | 899 | 18 | 0.93 | 0.86 |
Spain/Barcelona | 184,748 | 90 | 15 | 0.49 | 0.41 |
Spain/Navarra | 131,948 | 95 | 18 | 0.71 | 0.68 |
Spain/Valencia | 739,829 | 422 | 14 | 0.57 | 0.55 |
Switzerland | 176,318 | 190 | 18 | 1.07 | 0.83 |
USA | 616,892 | 617 | 24 | 1.00 | 0.98 |
Sociodemographic Factors
Race and Ethnicity
Non-Hispanic white women tend to have the highest incidence of DCIS in the US population, followed by black and Asian women, while Hispanic women have the lowest incidence (Fig. 1.3) [12]. This may be reflective of the screening behaviors of each of these racial and ethnic groups [13]. These national results have been echoed in other studies as well. Innos et al., for example, evaluated the trends in racial and ethnic differences in the incidence on DCIS in California in women ≥ 40 years, from 1988 to 1999. They observed an average annual age-adjusted incidence of DCIS of 45.3 per 100,000 in white women, 35.0 in black women, 30.9 in Asian-Pacific Islander women, and 21.8 in Hispanic women. Interestingly, while they found a steady increase in the incidence of DCIS in all racial/ethnic groups over the study period, Asian-Pacific Islander women were found to have experienced the steepest increase of a 9.1 % estimated annual percentage change, particularly in the age group 50–64 years in whom it was 12 % [14].
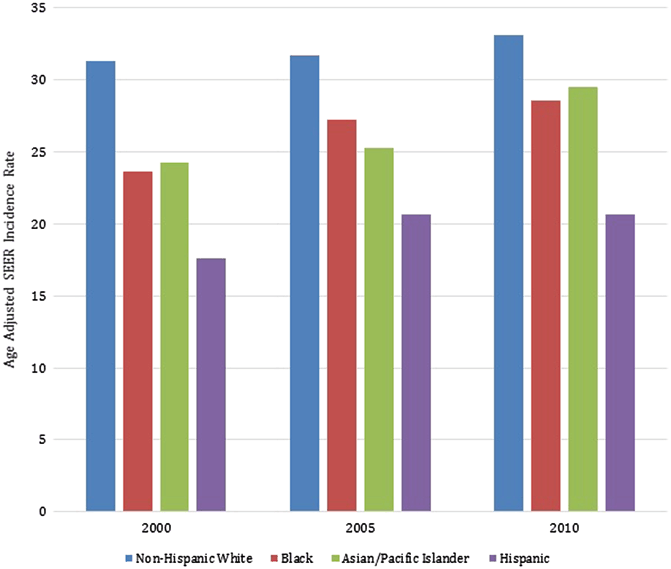
Fig. 1.3
Comparison of age-adjusted Surveillance, Epidemiology, and End Results (SEER) incidence rates over time by race/ethnicity (SEER 9)
Gender
While DCIS is primarily considered a disease of women, it can also occur in men. Anderson et al., in a review of in situ breast cancers reported in the SEER database from 1973 to 2001, found in situ carcinomas comprised 9.4 % of all male (280 of 2984) and 11.9 % of all female breast carcinomas (53,928 of 454,405). In situ rates rose 123 % for men and 555 % for women over this time period, perhaps due to screening mammography which would make it more detectable in women. Men also seem to be slightly older than women at diagnosis, with a median age of 62 years versus 58 years for their female counterparts [15].
Other Sociodemographic Factors
In addition to race/ethnicity and gender, other sociodemographic factors have also been linked to higher rates of DCIS. For example, there seems to be a higher incidence of DCIS in urban areas compared to rural areas, and the incidence of DCIS seems to be positively correlated, increasing socioeconomic status [16, 17]. It is plausible that these trends may be due to increasing use of mammography.
Clinicopathologic Features
In the broadest classification scheme, DCIS can be grouped into “comedo” and “noncomedo” types; the latter comprising various other subtypes including cribiform, solid, micropapillary, and papillary [18]. While the value of such classification schemes can be debated, particularly in the current era of genomic and molecular medicine, there have been a number of studies evaluating trends in the aggressiveness of DCIS cases diagnosed over time using these broad definitions. For example, Li et al. evaluated the trends in the incidence of comedo and noncomedo DCIS from 1980 to 2001, using the SEER database. Rates of noncomedo DCIS increased 6.1-fold (95 % CI: 5.7–6.5) over this time frame, while rates of comedo DCIS increased 15.7 times (95 % CI: 13.5–18.4) [19]. Pandya et al. evaluated the clinical and pathological characteristics of 204 DCIS patients treated before (1969–1985) and after (1986–1990) increased use of screening mammography. Between the two time periods, the incidence of comedo DCIS increased from 14 to 36 %, and grade 3 DCIS cases increased from 24 to 33 % [20].
Risk Factors
DCIS and invasive breast cancer have similar risk factors, suggesting a common etiology for both diseases [5].
Hereditary Factors
A first-degree family history of breast cancer is associated with an increased risk of DCIS, and this risk increases with the number of affected family members, particularly if there is a family history of breast cancer being diagnosed at a young age. In an analysis of the Million Women Study in the UK, Reeves et al. found no difference in the increased risk that a first-degree family history imparted for DCIS or invasive breast cancer (RR = 1.56 and 1.60, respectively) [21]. Reinier et al. also noted that the effect of family history also did not significantly vary in increasing the risk of premenopausal versus postmenopausal DCIS (RR = 1.9; 95 % CI: 1.2–2.8, and RR = 1.4; 95 % CI: 1.0–2.0 for pre- and postmenopausal women, respectively) [22]. Other studies have reported risk estimates ranging from 1.48 to 2.67 [23–25].
Claus et al. evaluated the BRCA 1 and BRCA 2 mutation status in 369 DCIS patients. They found, three patients (0.8 %) had mutations in BRCA 1 and nine (2.45 %) patients had mutations in BRCA 2, similar to rates found in patients with invasive breast cancer [26]. Similar to invasive cancers, mutation carriers also tend to present with DCIS at an earlier age than nonmutation carriers [27].
Reproductive and Hormonal Factors
In general, the reproductive factors that put women at risk of invasive breast cancer have a similar effect in increasing their risk of in situ disease. Several studies have found that the risk conferred by early menarche, parity, later age at first full-term pregnancy, and later menopause on the development of invasive cancer was the same as that for DCIS [21, 28, 29]. Interestingly, Reinier et al. found that nulliparity was more strongly associated with the development of DCIS than with invasive cancer [22]. While Meeske et al. found that parity reduced the risk of developing DCIS, they found no correlation between age at first full-term pregnancy and risk of DCIS [30]. Kabat et al., in evaluating data in the Women’s Health Initiative, found that only older age at menopause was associated with increased risk of developing DCIS; age at menarche, parity, and months of breast-feeding were not significant predictors [31]. Meeske et al., on the other hand, found that long duration of breast-feeding ( > 24 months) was associated with an increased risk of DCIS (odds ratio (OR) = 2.00; 95 % CI: 1.11–3.60) [30].
Oral contraceptive use has not been shown to increase the risk for developing DCIS [32, 33]. Studies by Longnecker et al. and Reeves et al. have shown an increased risk for DCIS with the use of hormone replacement therapy (OR = 1.60; 95 % CI: 1.00–2.58, and OR = 1.51; 95 % CI: 1.39–1.63, respectively) [21, 28]. Other studies, however, have not found a correlation between hormone replacement therapy and DCIS [29, 34].
Breast Density; Benign Breast Disease and Breast Biopsies
Patients with heterogeneously dense breasts and extremely dense breasts are at a higher risk for developing DCIS and invasive breast cancer . Gill et al. reported that women with a high breast density (≥ 50 %) were nearly three times as likely to develop DCIS compared to women with a low breast density (< 10 %) [35]. Two studies found an increasing risk of DCIS with increasing breast density; however, this risk was greater in premenopausal women [22, 36].
Personal history of benign biopsied breast disease is associated with an increased risk of DCIS . Trentham-Dietz et al. found that a personal history of benign biopsied breast disease was associated with twofold increased risk for developing DCIS (OR = 2.19; 95 % CI: 1.62–2.95). Interestingly, Weiss et al. found that the increased risk conferred by a previous benign breast biopsy was much greater for DCIS (adjusted RR = 1.99; 95 % CI: 1.2–3.0) than for local (adjusted RR = 1.23, 95 % CI: 0.9–1.7) and advanced disease (adjusted RR = 1.28; 95 % CI: 0.9–1.9).
Body Mass Index
Data regarding the impact of BMI on risk of DCIS are varied. While a number of studies have shown no association between DCIS and increasing body mass index (BMI) [22, 25, 34, 37], Kerlikowske et al. found that women with a BMI ≥ 35 had a significantly increased risk of DCIS compared to those with a normal BMI (OR = 1.46; 95 % CI: 1.14–1.87) [38]. Other studies, however, have found that, particularly in young women, there is a significant decrease in the risk of DCIS as BMI increases [28, 37]. While Longnecker et al. found this to be true in premenopausal patients (multivariate adjusted OR = 0.92; 95 % CI: 0.86–0.99), the same did not hold for the postmenopausal population (multivariate adjusted OR = 1.02; 95 % CI: 0.99–1.06) [28]. Others, however, who have similarly evaluated the pre- and postmenopausal populations separately, found no increased risk in either group [22].
Behavioral Risk Factors
Alcohol and Tobacco
A study by Trentham-Dietz et al. showed that alcohol consumption was associated with an increased risk for in situ disease (DCIS and lobular carcinoma in situ (LCIS), n = 291 patients). The OR among women who drank at least 183 g/week or two drinks/day was 2.34 (95 % CI, 1.32–4.16) compared to those who denied any alcohol intake [25]. However, a number of other studies have found no association between alcohol intake and risk of DCIS [23, 39, 40].
Similarly, there have been conflicting data regarding the impact of cigarette smoking on the risk of developing DCIS. One study found an inverse association between current smoking and risk of DCIS among women undergoing BCS [41]. Other studies, however, did not find an association of cigarette smoking with the risk of DCIS in postmenopausal women [42].
Exercise and Physical Activity
While a number of studies have found no association between physical activity and risk of DCIS [43, 44], one study found that, in women without a family history of breast cancer, an average exercise activity 0> 4 h/week was associated with a lower odds of DCIS than inactive women (OR = 0.53; 95 % CI: 0.34–0.82). This relationship, however, was not seen in women with a family history (OR = 2.29; 95 % CI: 0.62–8.22) [45].
Trends in Clinical Presentation and Detection
Prior to the introduction of national BCS programs, the incidence of DCIS was very low and nearly all diagnosed cases were symptomatic [1, 46] . Currently, DCIS accounts for 20–25 % of all newly diagnosed breast cancers and only 13–14 % of these cancers are symptomatic [47, 48]; the majority are screen detected. Barnes et al., in a series of 375 patients presenting with DCIS, noted that 82 % were screen detected. Symptomatic DCIS was more likely to be associated with an invasive component. Of those with pure DCIS presented symptomatically, the most common presentations were a breast mass (55 %), Pagets disease (13 %), nipple discharge (2.6 %), and a breast asymmetry (2.6 %) [48].
Since the introduction of screening mammography, the detection rates of DCIS have increased exponentially with the majority of cases being asymptomatic. Development of new, breast radiological techniques and improvement in physician expertise over the years, have further refined our ability to detect various benign and malignant breast pathology.
Screen film mammography (SFM) has been largely replaced by digital mammography (DM) over the past 15 years. Skaane, in a review of ten studies comparing SFM and DM, found that detection rates of DCIS were significantly higher with DM in three of the studies in comparison to SFM [49] .
Breslin et al. analyzed data from MarketScan Commercial Claims and Encounters Research Database for the years 2005 through 2008 and found a significant increase in MRI use for both DCIS and invasive breast cancer in women ≤ 65 years of age. In 2005, 22.8 % of patients underwent an MRI and by 2008, this proportion increased to 52.9 % [50]. Prior to 2000, breast MRI was considered a relatively poor imaging tool for DCIS. Three specific shifts in breast MRI occurred, which changed this assessment: (1) a shift from high temporal to high spatial imaging, revealing specific morphological features on MRI suspicious for DCIS; (2) increased use of MRI as a screening tool for high-risk patients, allowing more accurate comparisons of mammography versus MRI; and (3) improved understanding of features of non-mass-like malignant lesions, distinct from benign background parenchymal enhancement patterns [51] .
Menell et al. compared the ability of MRI and mammography to detect DCIS in a study of 39 sites of pure DCIS in 33 breasts of 32 women. Of 33 breasts involved, DCIS was discovered by MRI alone in 21 (64 %), by both MRI and mammography in 8 (24 %), and by mammography alone in 1 (3 %); in 3 breasts (9 %), DCIS was found at mastectomy without findings on mammography or MRI. MRI had significantly higher sensitivity than mammography for DCIS detection (29/33 = 88 % vs. 9/33 = 27 %, p < 0.001). Breast density was not found to impact these results [52]. Similarly, Kuhl et al. evaluated 7319 women who received both MRI and mammography for diagnostic assessment and screening. A total of 193 women were diagnosed with DCIS, 167 underwent both imaging tests preoperatively. A total of 93 (56 %) cases of DCIS were diagnosed by mammography and 153 (92 %) by MRI (p < 0.0001). Of the 89 cases of high-grade DCIS, 43 (48 %) were missed by mammography but were detected by MRI; MRI detected 87 (98 %) cases of high-grade DCIS [53] .
While MRI may be successful in detecting DCIS, its utility in screening is limited and it is cost prohibitive. Further, there are limited data that MRI results in improved long-term outcomes. In a recent study by Pilewskie et al., MRI resulted in additional biopsies in 38 % of patients with DCIS (vs. 7 % in patients who did not have an MRI), yet the rate at which these biopsies yielded a cancer diagnosis was not significantly different between those who had an MRI and those who did not (26 % vs. 33 %, respectively, p = 0.73). In addition, MRI tended to overestimate the size of the DCIS , with 43.9 % of DCIS lesions being overestimated by at least 1 cm in the MRI group [54]. A number of studies have noted that MRI results in an increased rate of mastectomy; [55, 56] and in those who underwent breast-conserving surgery , use of perioperative MRI has not been shown to be associated with a lower locoregional or contralateral recurrence rate [57].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree