1 Epidemiology
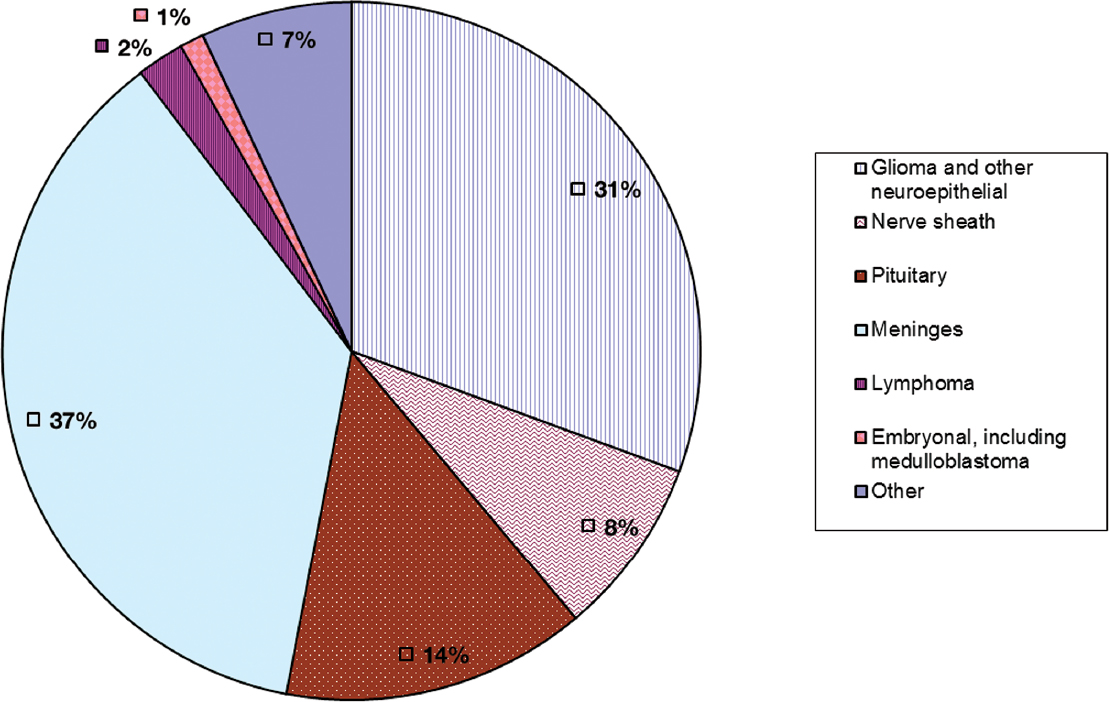
In the United States, 70,000 new cases of primary malignant and benign brain and central nervous system (CNS) tumors are diagnosed each year, and 14,000 patients die; 31% of these tumors are gliomas and 37% are meningiomas.1 There are two main types of epidemiological studies that contribute to our understanding of brain tumors: descriptive studies characterize the incidence of brain tumors and the mortality and survival rates associated with them in a given population by person, place, and time; analytic studies either compare the risk of brain tumors in people with and without certain characteristics (cohort studies) or, more commonly, because of the relative rarity of brain tumors, compare life histories of people with and without brain tumors (case-control studies). The main purposes of epidemiological studies are to characterize the distribution of brain tumors and understand their underlying causes. The distribution of these tumors may yield clues to their causes or point to high-risk populations requiring enhanced surveillance.
 Descriptive Epidemiology
Descriptive Epidemiology
Descriptive epidemiological studies show variation in brain tumor incidence and mortality by time, geographic region, ethnicity, age, gender, histologic type, and intracranial site.
Although primary brain tumors are relatively rare compared with metastatic brain tumors or more common primary cancer sites such as lung, breast, prostate, and colorectal, they constitute an important source of morbidity and mortality. Fig. 1.1 shows the percentages of brain tumors by major histological types.
Special Consideration
• In children, brain tumors cause one fourth of all cancer deaths. The overall annual incidence rate of primary malignant and benign brain and CNS tumors in the United States is approximately 21 per 100,000 individuals, and for primary malignant brain tumors it is approximately 7 per 100,000.
Special Consideration
• The Central Brain Tumor Registry of the United States (CBTRUS; www.cbtrus.org), formed in 1994, reports incidence rates and the estimated number of new cases for all primary malignant and nonmalignant tumors of the CNS, including brain and spine, pituitary and pineal glands, and olfactory tumors of the nasal cavity. The CBTRUS compiles incidence and survival data from the Surveillance, Epidemiology, and End Results Program (SEER; seer.cancer.gov) and the National Program of Cancer Registries (NPCR; http://www.cdc.gov/cancer/npcr/). Following passage of the Benign Brain Tumor Cancer Registries Amendment Act, all U.S. cancer registrars began recording benign tumors of the brain and CNS beginning in 2004. The American Cancer Society (ACS; www.cancer.org) estimates new cases of and deaths caused by primary malignant tumors of the brain and CNS, excluding lymphoma, leukemias, tumors of the pituitary and pineal glands, and olfactory tumors of the nasal cavity. The World Health Organization’s International Agency for Research on Cancer (IARC; www.iarc.fr) reports worldwide incidence rates of primary malignant tumors of the brain and CNS, excluding lymphoma, leukemias, tumors of the pituitary and pineal glands, and olfactory tumors of the nasal cavity.
Time Trends in Incidence and Mortality
Time trend studies report an increase in brain tumor incidence over the past three decades, with improved reporting, increased use of diagnostic imaging, and changing attitudes toward diagnosis in the elderly held responsible for much of the observed increase.2 However, some researchers also suggest that the overall increase (especially among children) may be due to changes in etiologic factors.
Ethnic and Geographic Variation in Incidence and Mortality
Interpretations of ethnic and geographic variation in the occurrence of brain tumors are complicated by problems in ascertainment and reporting. Regions with the highest reported rates of primary malignant brain tumors (e.g., Northern Europe, the U.S. white population, and Israel; rates of 11 to 20 per 100,000 people) generally have more accessible and developed medical care than areas with the lowest rates (e.g., India and the Philippines; rates of 2 to 4 per 100,000 people).3 However, some of the variation suggests ethnic differences in inherited susceptibility or cultural or geographic differences in risk factors.4,5 For example, the rate of malignant brain tumors in Japan, an economically prosperous country, is less than half the rate in Northern Europe. In the United States, whites have higher rates of glioma than blacks but lower rates of meningioma; this would be difficult to attribute solely to differences in access to medical care or diagnostic practices in the two groups.1
The absolute variation in the occurrence of brain tumors between high-risk and low-risk areas is on the order of fourfold, compared with the 20-fold difference observed for lung cancer or the 150-fold difference observed for melanoma.3 Thus, it seems unlikely that strong geographic risk factors for brain tumors exist in the same manner that they do for other neoplasms (e.g., cigarette smoking and asbestos exposures for lung cancer, sunlight intensity for melanoma).
Fig. 1.1 Percentages of primary brain tumors by histological type. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 21)
Age and Gender Variation in Incidence and Mortality
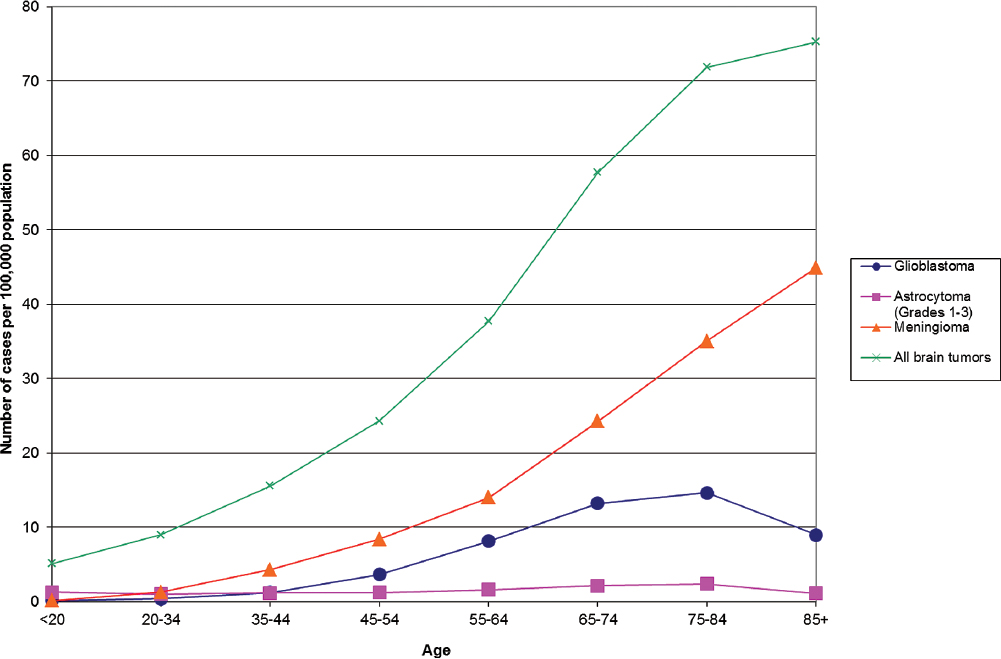
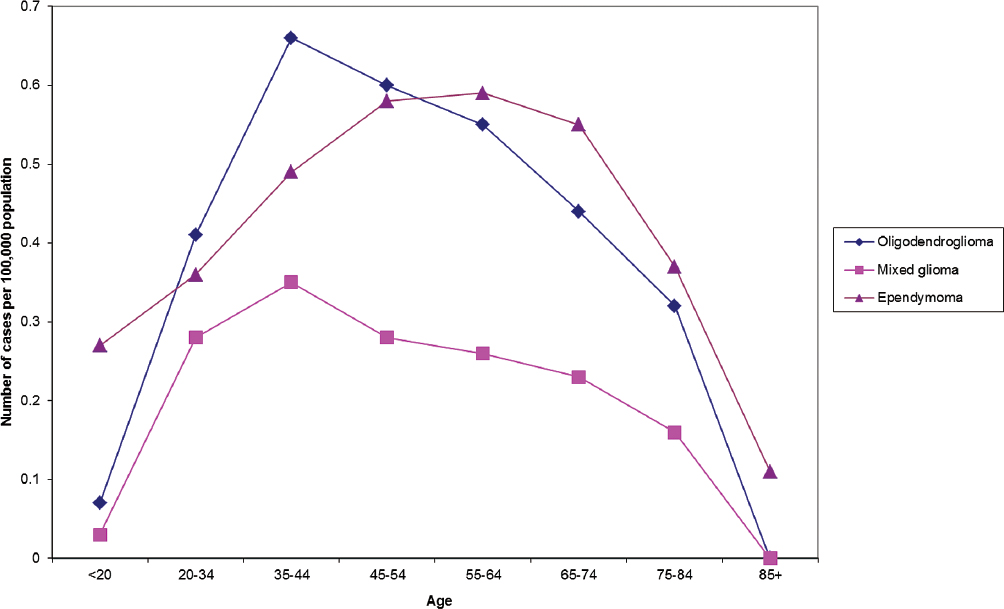
Overall, the median age of onset for primary brain tumors is 59, and the median ages of onset for glioblastoma and meningioma are 64 and 65, respectively.1 The age distributions of primary brain tumors vary by site and histological type (Figs. 1.2, 1.3, 1.4). As with other types of cancer, the increased incidence of most types of brain tumors with age could be due to length of exposure required for malignant transformation, the necessity of many genetic alterations prior to the onset of clinical disease, or diminished immune surveillance. Interestingly, there is a decline in the incidence of glioblastoma and astrocytoma among those 85 and older (Fig. 1.2), whereas the incidence of oligodendroglioma and ependymoma peak in middle age (Fig. 1.3).
Pearl
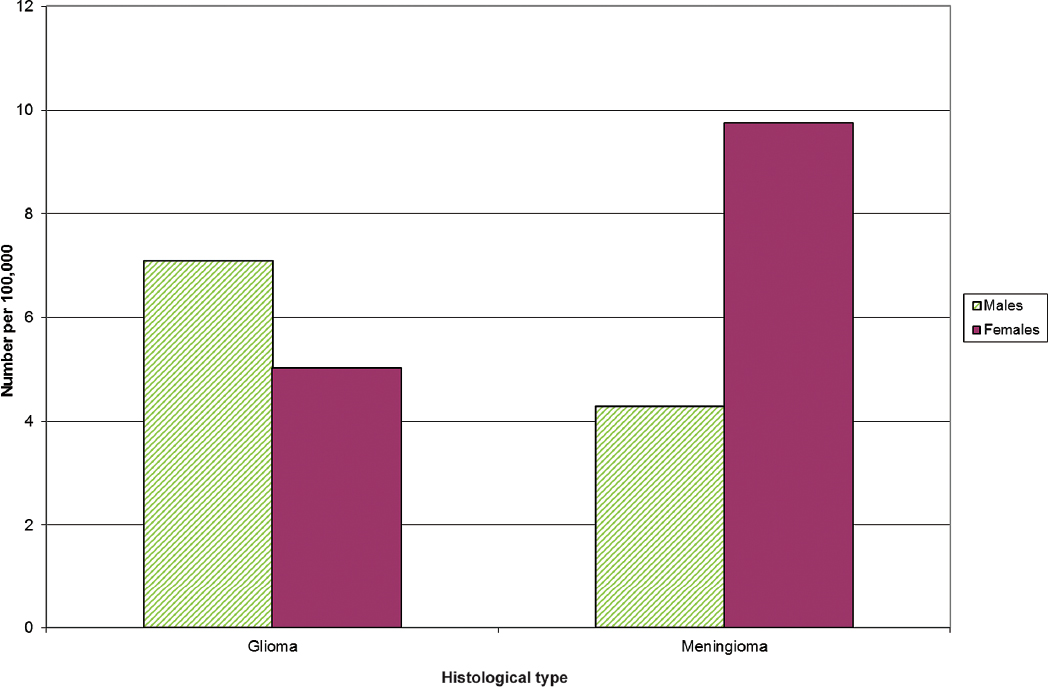
• Among the most consistent finding in the epidemiology of brain tumors is the difference in incidence rates between genders; glioma is more common in men and meningioma is more common in women.
Fig. 1.2 Primary brain tumor incidence rates by age at diagnosis for all and most common histological types. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 121)
Fig. 1.3 Primary brain tumor incidence rates by age at diagnosis of oligodendroglioma, mixed glioma, and ependymoma. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 121)
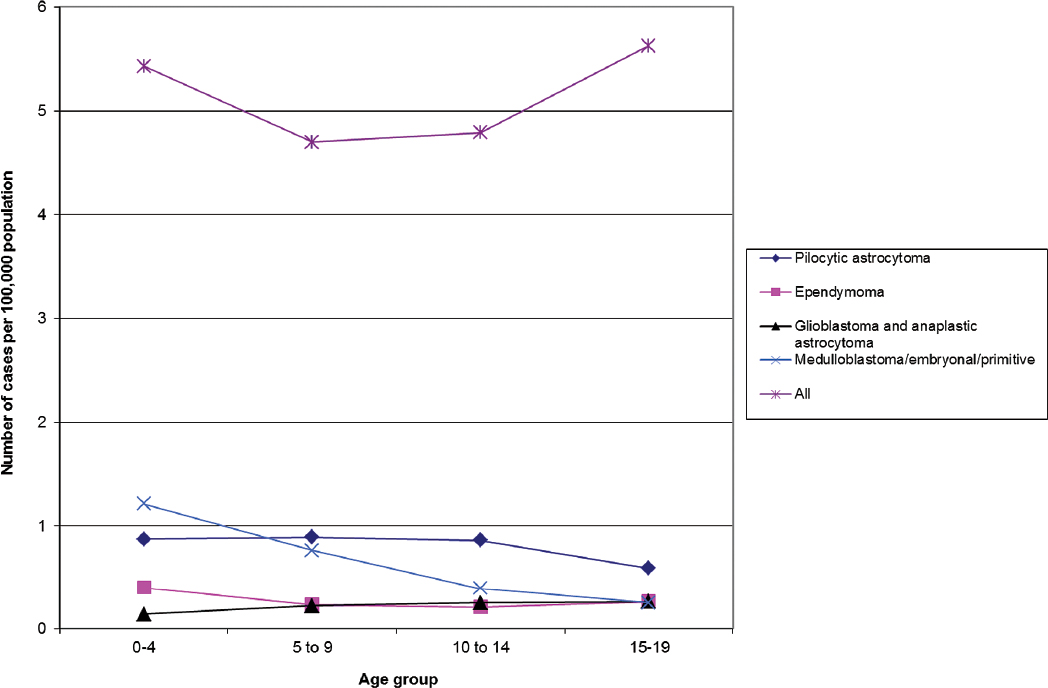
Fig. 1.4 Childhood primary brain tumor incidence rates by age and histologic type. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 161)
Glioma rates are higher in males and meningioma rates are higher in females (shown for U.S. data in Fig. 1.5). Because of the consistency of this finding across almost all ages and populations studied, a comprehensive theory of brain tumor etiology should account for this fact. However, this important epidemiological observation remains unexplained.
Survival and Prognostic Factors
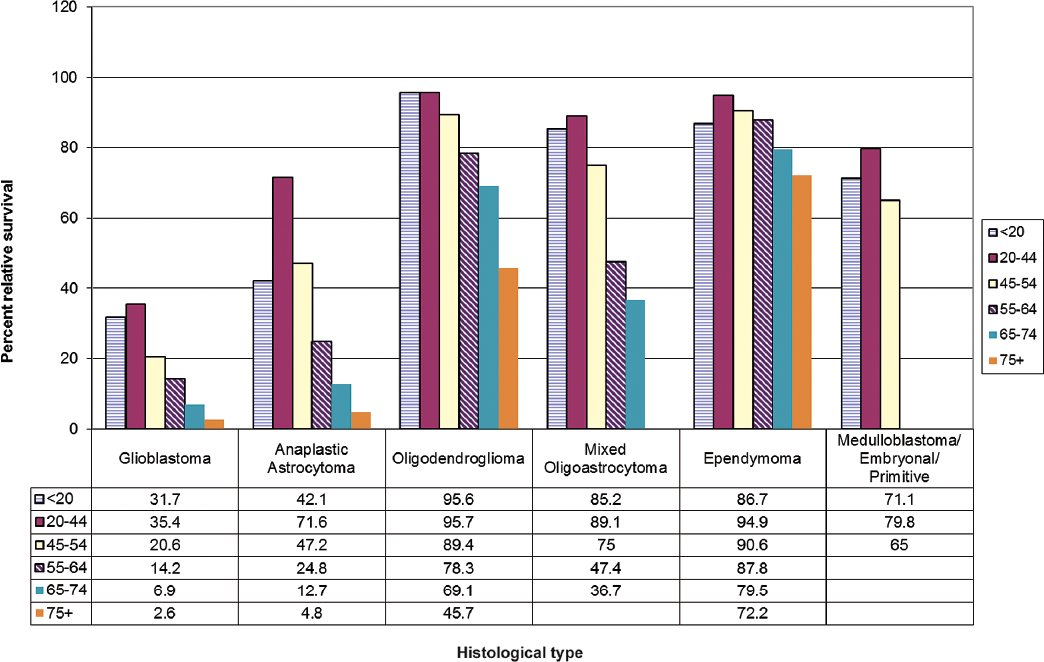
For individuals with primary malignant brain tumors, histological type and age are strong prognostic factors (Fig. 1.6). In addition, grade of tumor, extent of lesion resection, tumor location, administration of radiotherapy (high-grade tumors only), and some chemotherapy protocols have been consistently linked with survival in both population registry and clinical trial data.1,6 The Radiation Therapy Oncology Group and other clinical trial groups provide useful information on prognostic factors from patients whose pathological features have been reviewed and who are treated in clinical trials. However, many patients do not enter clinical trials, and thus these trial results may not be representative of the general population of patients with glioma.
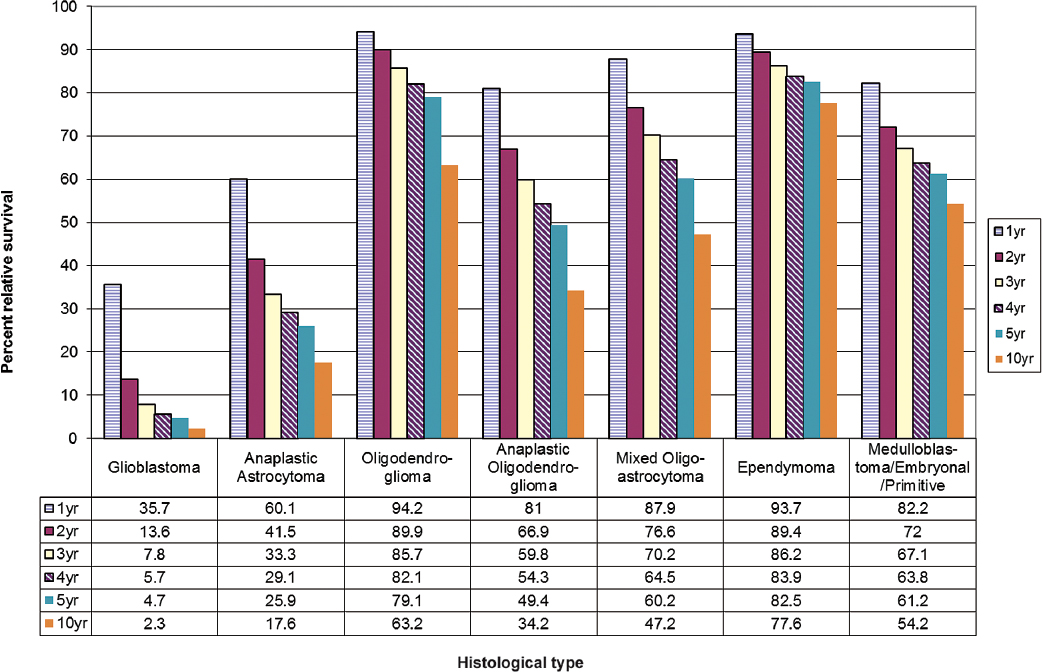
One study examined changes in glioblastoma survival rates by age, race, and gender for the time period from 1993 to 2007.7 A modest improvement in survival of 2 to 4 months was observed following widespread adoption of temozolomide treatment around 2005. No improvement in survival was observed for patients in the oldest age groups (80+). Fig. 1.7 shows 1- to 10-year percent relative survival for glioblastoma and other common histologies.
Molecular Markers of Survival/Prognosis
The role of molecular markers in prognosis is a growing area of investigation that has led to some important findings. Combined losses of chromosomes 1p and 19q in oligodendroglial tumors are favorable prognostic indicators, as are mutations of the genes IDH1 and IDH2.8 A genetic signature often observed in oligodendrogliomas, consisting of mutations in IDH1/2, CIC, and FUBP1, correlates with improved overall survival. In astrocytic tumors, mutations in IDH and ATRX correlate with improved survival compared to astrocytic tumors without these mutations; however, these patients still experience poorer survival than patients with IDH-mutated oligodendroglioma.9
A large prospective trial of patients with newly diagnosed glioblastoma indicated that methylation of the MGMT promoter is a marker of improved outcome.6 Interestingly, MGMT methylation appeared to be more strongly associated with survival among patients who received temozolomide than among those who did not, raising the possibility that MGMT methylation may be a predictive marker of response to this alkylating agent. A genome-wide glioma-CpG island methylator phenotype (G-CIMP) has also been linked to improved survival in glioma patients. This hypermethylator phenotype is also associated with IDH mutations, indicating that there is overlap of glioma prognostic markers.8
Fig. 1.5 Primary brain tumor incidence rates age-adjusted to the 2000 U.S. standard population by gender for gliomas and meningiomas. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 91)
Fig. 1.6 Two-year relative survival rates for primary malignant brain tumors by age group. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 221)
Fig. 1.7 One-, 2-, 3-, 4-, 5- and 10-year relative survival rates for primary brain tumors. (Adapted from Central Brain Tumor Registry of the United States, 2005–2009, Table 211)
A recent genome-wide association study of single nucleotide polymorphisms (SNPs) evaluated in similarly treated glioblastoma patients indicates that heritable variation in a gene called SSBP2 may impact patient survival.10 Another recent study indicated that somatic mutations in the TERT promoter are associated with poorer survival experiences among glioblastoma patients.11 Both these findings await further validation.
Tumor Heterogeneity
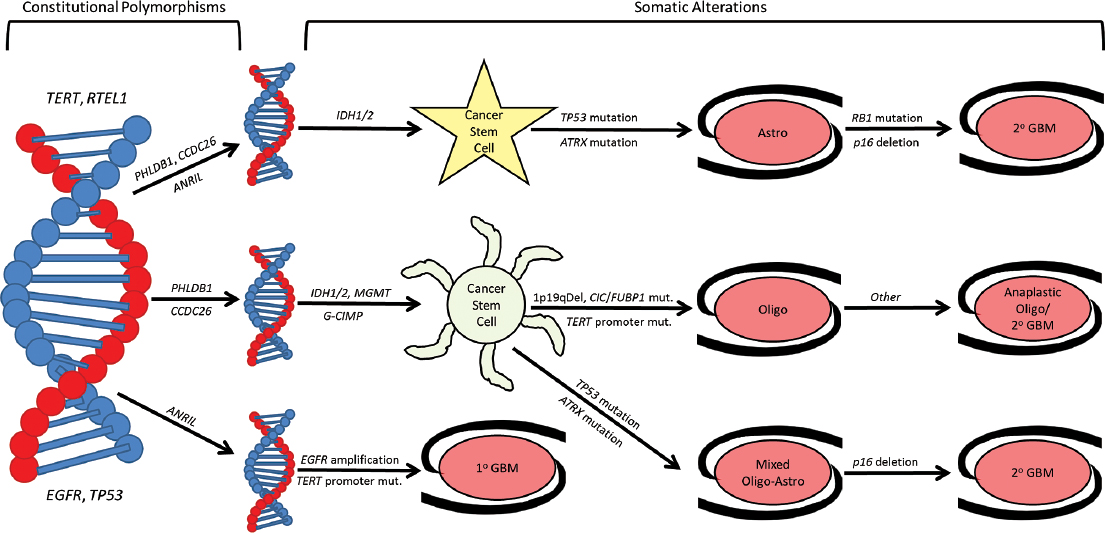
Heterogeneity among tumors of varying histology and anatomic location has long been recognized. Cytogenetic and molecular studies show tumor heterogeneity exists within single histological categories. There are now several genetic and molecular changes thought to potentially cause primary CNS tumor formation.9 Glioblastoma may arise by two pathways that can be defined in clinical terms: the first pathway results from tumor progression from lower grade astrocytoma, whereas a second pathway has no clinically evident precursor (i.e., de novo glioblastoma). IDH mutation, TP53 mutation, and EGFR amplification appear to correlate with these clinical classifications (Fig. 1.8).
Primary glioblastoma is likely to harbor EGFR amplification, whereas secondary glioblastomas, arising from lower grade precursors, are more likely to harbor TP53 and IDH mutations. The same appears true of somatic mutations in TERT and ATRX; the former is associated with primary glioblastoma, whereas the latter is found more commonly in lower grade astrocytomas and secondary glioblastomas.11 Although this distinction is not absolute, it raises the possibility that distinct subtypes of glioblastoma, although similar histologically, may display substantial clinical differences.
Some important modifications that occur in 5 to 40% of glioblastoma and anaplastic astrocytoma include EGFR amplification and mutation, amplification of CDK4 or MDM2, and deletion or mutation of TP53, CDKN2A, RB1, PTEN, TERT, or ATRX. In 30 to 40% of astrocytomas, TP53 is deleted or mutated.8 Chromosomes 1p and 19q are deleted in 40 to 90% of oligodendrogliomas. These tumors also frequently carry mutations in FUBP1 (on chromosome 1p) and CIC (on 19q), as well as IDH1 or IDH2 mutation.8 Chromosome 22 is deleted in 25 to 50% of ependymomas. Varying proportions of medulloblastomas display amplification of MYCN and CMYC, deletion or mutation of PTCH, or deletion of chromosome 17p. About 20 to 30% of pilocytic astrocytomas have deletion of chromosome 17q. NF2 is deleted or mutated in > 50% of meningioma or schwannoma, and VHL is deleted or mutated in about 15% of hemangioblastomas. Approximately 5 to 25% of non-NF2 mutated meningiomas also carry mutations in TRAF7, KLF4, AKT1, or SMO.12 This emphasizes the enormous heterogeneity of molecular modifications within and between histological types of primary brain tumors.
 Risk Factors
Risk Factors
There are few established environmental risk factors for brain tumors, in part due to tumor heterogeneity and extended time since exposure. Table 1.1 summarizes risk factors for adult and childhood gliomas, and Table 1.2 does the same for meningiomas and other CNS tumors. Several excellent and comprehensive reviews of the epidemiological literature on primary brain tumors provide more detailed information about these factors.2,13–16
 Genetic and Familial Factors
Genetic and Familial Factors
Hereditary Cancer Syndromes
The heritability of brain tumors is suggested by reports of these tumors occurring more commonly in individuals with hereditary syndromes. Many studies have attempted to identify rare genetic mutations conferring increased brain tumor risk within families.17 Although such methods can identify genes contributing to brain tumor risk in families with rare mendelian diseases, these genes explain only a small proportion of brain tumor incidence at the population level. Common familial exposure to environmental carcinogens may also contribute to the induction of some brain tumors.18 Although Li-Fraumeni syndrome (LFS) is the familial tumor syndrome most frequently associated with glioma, numerous other rare mendelian disorders also increase glioma risk. These syndromes are listed in Table 1.1 (for glioma) and Table 1.2 (for meningioma and other CNS tumors).
Familial Aggregation
Because only a small proportion of brain tumors are attributed purely to mendelian disorders, the remaining hereditary risk is likely associated with low-penetrance genetic variants and the effects of shared environment.18 Several case-control studies suggest familial aggregation of primary brain tumors.17,19 Segregation analyses of more than 600 adult glioma patients’ families found a polygenic model best explained the pattern of brain tumor incidence.20 Genome-wide association studies of common genetic variants, which contribute modest brain tumor risk, explains some of the missing heritability in brain tumor susceptibility.
Genome-Wide Association Studies
Genome-wide association studies (GWASes) have had far greater success in revealing the genetic etiology of primary brain tumors than previous candidate-gene studies.16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree