| Percentage for 1975/2003 by infection site | ||||
|---|---|---|---|---|
| Pathogen | Bloodstream | Woundsa | Pneumonia | Urine |
| Enterococci | 8.1/14.5 | 11.9/13.9 | 3.0/1.3 | 14.2/17.4 |
| S. aureus | 16.5/14.3 | 18.5/22.5 | 13.4/27.8 | 1.9/3.6 |
| CoNS | 10.3/42.9 | 7.4/15.9 | 2.6/1.8 | 3.2/4.9 |
| Other species | 8.0/4.5 | 8.8/5.8 | 6.9/3.2 | 2.2/1.2 |
a Skin and skin structure infections (SSSI).
Abbreviations: CoNS = coagulase-negative staphylococci; NNISS = National Nosocomial Infections Surveillance System; CDC = Centers for Disease Control and Prevention.
Cephalosporins are generally not active nor bactericidal against enterococci, and they may therefore result in a selective advantage for this organism. Fluoroquinolones are also only modestly active against these species. Enterococcus faecalis produce most human enterococcal infections (70% to 80%), and Enterococcus faecium accounts for most (10% to 16%) of the remainder. However, E. faecium may account for a greater proportion (>30%) of enterococcal bacteremias (see Table 135.2). Antimicrobial resistance is a particular problem among E. faecium isolates. Other species of interest are Enterococcus casseliflavus and Enterococcus gallinarum, not because of the frequency with which they are isolated (<5%), but because of the intrinsic low-level resistance to vancomycin (e.g., the vanC genotype and resultant generally intermediate susceptibility phenotype; minimum inhibitory concentrations [MICs], 4–8 μg/mL).
| Vancomycin nonsusceptible rate (%VanA)a | ||
|---|---|---|
| Surveillance year | E. faecium | E. faecalis |
| (no. tested) | (3018) | (5671) |
| 2000 | 57.1 (84.2) | 4.0 (42.5) |
| 2001 | 60.0 (88.2) | 1.4 (35.7) |
| 2002 | 70.7 (86.4) | 2.9 (55.2) |
| 2003 | 70.5 (88.5) | 4.6 (34.8) |
| 2004 | 68.6 (94.9) | 2.6 (50.0) |
| 2005 | 70.5 (95.6) | 4.3 (51.2) |
| 2006 | 69.7 (99.0) | 4.8 (87.5) |
| 2007 | 71.6 (97.6) | 3.9 (89.7) |
| 2008 | 75.3 (97.9) | 6.5 (75.4) |
| 2009 | 76.3 (97.6) | 3.2 (65.6) |
| 2010 | 80.7 (97.0) | 5.3 (77.4) |
| 2011 | 77.7 (96.6) | 4.1 (82.9) |
a A total of 401 other Enterococcus spp. were tested (data not shown) for a total of 9090 US enterococcemias.
In addition to the problems posed by the increasing frequency of enterococcal infection, the therapy of these infections has become challenging as resistance to ampicillin, high-level resistance to aminoglycosides (preventing bacteriocidal combination therapy), and glycopeptides (vancomycin and others) have occurred; emerging linezolid (an oxazolidinone), daptomycin, tigecycline (a glycylcycline), and quinupristin/dalfopristin resistance have also narrowed the number of therapeutic regimens. In addition, the risk of failure of trimethoprim–sulfamethoxazole (TMP–SMX) in therapy even for urinary tract infection has become recognized regardless of in vitro susceptibility where some standard organizations recommend not testing TMP–SMX. All enterococci are intrinsically resistant to achievable in vivo levels of aminoglycosides; however, synergic killing can occur when aminoglycosides are combined with a cell wall-active agent such as a penicillin or a glycopeptide. Strains resistant to high levels of aminoglycoside (>500 μg/mL of gentamicin or >1000 μg/mL of streptomycin) are not susceptible to the synergic co-drug effects of the aminoglycosides. It is clinically significant that cross-resistance to the synergic activity of the aminoglycosides is not complete between gentamicin (and the related compounds tobramycin, netilmicin, amikacin, kanamycin, and isepamicin) and streptomycin. Streptomycin may be used successfully in combination to treat some high-level gentamicin-resistant strains. The selection of the appropriate aminoglycoside co-drug should be directed by validated in vitro susceptibility tests and the availability of streptomycin for clinical use.
Resistance to vancomycin is much more common with E. faecium isolates than with E. faecalis. Reports in the United States in the late 1990s suggested that the overall vancomycin-resistant rate for enterococci was approximately 20% among bloodstream infections, and higher resistance rates for some other drugs and species limited therapeutic choices. These resistance rates have escalated through 2011 (Table 135.2). Acquired vancomycin resistance is often associated with resistance to teicoplanin (Van A phenotype or vanA gene) or may occur in the absence of cross-resistance to teicoplanin (Van B phenotype or vanB gene). This difference can be clinically significant in nations where teicoplanin has been clinically available since the 1980s, and could also be relevant in the United States as telavancin and dalbavancin (long-acting lipoglycopeptide) are marketed worldwide. Also, another investigational glycolipopeptide, oritavancin, retains activitity against VanA and VanB resistance phenotypes, as does daptomycin. Willems and colleagues have identified a hospital-adapted E. faecium clone (CC-17) that has evolved ampicillin-, vancomycin-, and fluoroquinolone-resistant patterns. This CC-17 has been documented in vancomycin-resistant enterococci (VRE) populations in the United States (Figure 135.1) and since 1999 in Europe has progressively increased at rates comparable to those in the United States in the earlier years (1990s). Vancomycin resistance in E. faecium bacteremia isolates (SENTRY Program, 2008–2011, Table 135.2) now approaches 80%, dominantly VanA patterns.
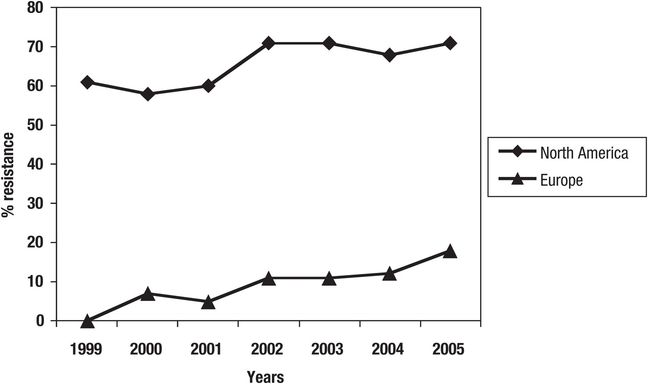
Figure 135.1 Progression of vancomycin resistance in E. faecium among bloodstream isolates studied in North America and Europe, including presence of isolates with antibiograms consistent with those of CC-17 (SENTRY Program, 1999–2005).
For the clinician, the problems posed by the emergence of resistance have been exacerbated by the technical difficulties in reliable detection of these resistances. In vitro resistance to TMP–SMX as a result of the ability of the most prevalent enterococci to use thymidine or thymine in the susceptibility test medium (escapes bactericidal action) has been addressed by use of media free of or low in concentration for these antagonists. However, there can be significant amounts of antagonists in the urine, and therefore the meaning of in vitro test results performed in these “improved” test conditions is compromised. Routine testing against ampicillin without testing for organism β-lactamase production may result in false-susceptible results. However, β-lactamase production continues to be an exceedingly rare mechanism of resistance (≤0.1%) among E. faecalis isolates. A number of problems with the detection of high-level aminoglycoside resistance using the most prevalent automated and commercial broth microdilution susceptibility test systems (Vitek 2, MicroScan, BD Phoenix) have been reported, although in some cases these appear to have been resolved. Similarly, with vancomycin and other newer agent resistances reported from both automated susceptibility test systems, Etest and disk diffusion test interpretive criteria may continue to require modifications over time to enable consistent, accurate detection of resistant strains. Some newer agents now necessitate testing with surfactants (polysorbate-80) to prevent binding to plastic reagent panels.
Highly reliable, empiric therapy of enterococcal infection is not consistently satisfactory given the complexity and variability of resistance patterns; and therefore, availability of prompt, reliable susceptibility test results is critical. At present, disk diffusion susceptibility testing, Etest, Vitek or BD Phoenix Systems, and the reference broth microdilution or agar dilution methods are reliable methods for detection of important resistances. However, recent reports of false resistance have been reported for some newer agents, including linezolid, caused by fuzzy zone diameter edges and trailing end points by dilution test methods.
Enterococcal bloodstream infection
Isolation of enterococci from blood
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





