13 Endoscopic Approaches
Over the last two decades, the use of the endoscope has become increasingly popular for approaching skull base and intraventricular tumors, as well as for serving as an adjunct and alternative for other intracranial tumor microsurgery. The endoscope increases the field of view of the microscope by advancing the lens and light source into the surgical cavity, permitting visualization around corners. Several factors have contributed to the growth and success of endoscopic surgery, including advances in available fiber optic equipment and specialized surgical instruments, as well as the fostering of collaborative efforts between rhinologic and neurologic surgeons.
 Endoscopic Endonasal Skull Base and Pituitary Surgery
Endoscopic Endonasal Skull Base and Pituitary Surgery
Intracranial tumors involving the skull base have been traditionally approached via transfacial and transcranial surgery and can be associated with relatively high morbidity and long recovery times. Although these approaches offer wide exposure and working space, performing brain retraction, neurovascular manipulation, sinus obliteration, and wound healing, and maintaining good cosmesis remain significant issues. The endoscope has enabled the transsphenoidal approach, as well as the advent of extended approaches, to serve as less invasive ways to manage a variety of intra- and extracranial neoplasms. The keys to performing successful endoscopic skull base surgery include an experienced surgical team, appropriate instrumentation, adequate operative resources (e.g., neuro-navigation), and careful case selection. Stereotactic navigation is a standard in all endoscopic endonasal skull base surgeries.
The importance of careful case selection cannot be emphasized enough and is critical to ensuring the success of the operation. Pathology extending laterally over the orbits or behind and lateral to the carotid arteries, for instance, can be quite difficult to remove even with extended approaches. Likewise, lesions extending into or just behind the frontal sinus can prove difficult to reach even with angled scopes, and the closure of the resulting defects can be quite challenging. Although cavernous sinus invasion is not an absolute contraindication, it warrants careful preoperative evaluation of surgical goals. The surgeon may elect to enter the cavernous sinus to resect the tumor using a safer approach medial and posterior to the carotid artery or a riskier lateral and anterior approach, understanding the additional risks to the neurovascular contents, or may opt for an intentional subtotal resection with planned stereotactic radiotherapy postoperatively, depending on the pathology. Finally, the differential diagnosis for masses of the sella can be quite vast, including pathology that would not benefit from endonasal surgery such as large cavernous segment aneurysms and exquisitely radiosensitive tumors. Such lesions can often be discovered preoperatively and may require a very different workup.
Pearl
• Successful endoscopic endonasal surgery relies on an experienced team of collaborative surgeons, available state-of-the-art operative resources, and appropriate case selection and preoperative evaluation.
The endonasal skull base approaches can be classified based on which nasal sinus is opened to access the pathology and the ultimate target to be reached.1 The available sinuses are the sphenoid, ethmoid, maxillary, and frontal. Each sinus can be utilized to access a different region of the midline and paramedian skull base, and these corridors can also be combined together for larger, multicompartmental tumors (Table 13.1). In addition, it is possible to merge the endonasal skull base approaches with the transcranial approaches for large multicompartmental tumors in either a combined or staged manner.
Transsphenoidal Corridor
The transsphenoidal corridor can be used to access the sella for pituitary tumors and small Rathke cleft cysts, and can also be extended superiorly, inferiorly, and laterally to reach the suprasellar cistern, the top one third of the clivus, and the medial cavernous sinus and medial optic canal, respectively. With these extended approaches, the range of suitable pathology increases to include craniopharyngiomas, meningiomas, chordomas, and chondrosarcomas. Pathology in this region can often remain clinically silent or cause symptoms of local mass effect on the brain or optic apparatus, commonly resulting in hypothalamic-pituitary dysfunction and visual deficits. Pre-operative endocrinologic and ophthalmologic assessments are imperative in patients with parasellar pathology.
Table 13.1 Endonasal Skull Base Approaches
| Corridor | Approach | Target |
| Transsphenoidal | Transsellar Transplanum, transtuberculum Transclival Transcavernous Transcanalicular | Pituitary gland Suprasellar cistern Upper one third of clivus Medial cavernous sinus Medial optic canal |
| Transnasal | Transcribriform Transclival Transodontoid | Olfactory groove Lower two thirds of clivus and petrous apex Craniovertebral junction |
| Transethmoidal | Transfovea ethmoidalis Transorbital Transsphenoidal | Anterior fossa Medial orbit Lateral cavernous sinus |
| Transfrontal | Transfrontal | Anterior fossa |
| Transmaxillary | Transpterygoidal | Pterygopalatine fossa Infratemporal fossa Lateral sphenoid sinus Lateral cavernous sinus Meckel’s cave |
Transsellar Approach
The transsellar approach is reserved for pituitary microadenomas, macroadenomas with minimal suprasellar or cavernous invasion (generally < 2.5 cm in diameter), and intrasellar Rathke cleft cysts. For intrasellar pathology, endoscopic endonasal approach (EEA) results are generally no better than those reported using transsphenoidal microscopic techniques, because the pathology is directly in front of the surgeon and the increased field of view offers no significant advantage. This is particularly true for microadenomas. A large review of 200 patients with micro- and macroadenomas demonstrated comparable rates of gross total resection (GTR) as measured against large historical microscopic series.2 However, several studies have demonstrated that the use of endoscopy enables additional pituitary tumor removal following microscopic-assisted removal, indicating a clear role in increasing the extent of resection.3 Similarly, for macroadenomas, the EEA clearly appears to increase the extent of resection.
Because the majority of hormone-producing adenomas are small, the results of EEA for hormone restoration are comparable to those achieved with a microscope. However, a study of 120 patients with functioning adenomas found the hypersecretion remission rate was significantly better in the EEA group (63%) than in the microsurgical group (50%).4 Similarly, another large series reported 71% biochemical cure in growth hormone (GH)-secreting adenomas, in addition to 81% and 88% remission rates in Cushing’s disease and prolactinomas, respectively.5 Approximately 80 to 100% of patients with prolactinomas can be afforded endocrinologic remission (i.e., postoperative prolactin levels < 20 ng/mL in females or < 15 ng/mL in males).6 Approximately 52 to 84% of patients with acromegaly can also achieve endocrinologic cure with surgery.6
The EEA for Rathke cleft cysts (RCCs) has also shown excellent results. One study reported two recurrences in 23 symptomatic patients who underwent cyst drainage and partial wall resection.7 All patients with visual impairments improved postoperatively with half of the patients experiencing improvement in preoperative pituitary dysfunction as well. Although the transsphenoidal approach currently remains the preferred approach for symptomatic RCCs, whether or not removal of the cyst wall is necessary remains debatable. Cyst wall excision increases the rate of diabetes insipidus and hypopituitarism but may decrease rates of recurrence when done safely.
Transplanum, Transtuberculum Approach
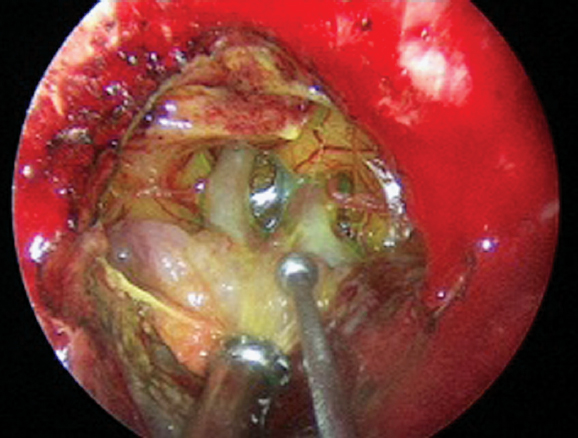
The transplanum, transtuberculum approach can be used to expose the suprasellar cistern to remove giant macroadenomas with significant suprasellar extent (> 1 cm above the jugum), as well as craniopharyngiomas and planum and tuberculum meningiomas. Harvesting a nasoseptal flap at the beginning of the operation is important because significant cerebrospinal fluid (CSF) leak is expected (see below). Placement of a lumbar drain at the start of the procedure has been shown to significantly prevent intraoperative CSF leaks.8 Case selection is especially critical because tumors that extend lateral to the anterior clinoids and > 1 cm past the lateral wall of the carotid artery may not be suitable for EEA. Some practitioners have cautioned against endonasal resection of meningiomas that encase arteries or that are without evidence of a cortical cuff between the tumor and the anterior communicating artery,9 but these are relative contraindications and can be overcome with advanced bimanual microsurgical dissection technique (Fig. 13.1). Another potential indication is the “hypophyseal transposition” or “hypophysopexy,” whereby the pituitary gland is mobilized with a fat graft away from unresectable tumor in the cavernous sinus to protect the gland from the deleterious effects of stereotactic radiosurgery.10
Giant pituitary macroadenomas have generally been defined as > 4 cm in diameter, and more recently a 10 cm3 volumetric definition has been used.11 A recent meta-analysis found significantly higher rates of GTR in patients with macroadenomas who underwent EEA resection compared to those who underwent transcranial or transsphenoidal microscopic surgery.12 Specifically, GTR was achieved in 9.6% of patients undergoing open transcranial resection, with 82% of patients experiencing complications including permanent diabetes insipidus (9.1%), hypopituitarism (9.1%), CSF leak (7.1%), and cerebral infarcts (6.1%).12 Conversely, GTR was achieved in 47.2% of patients undergoing EEA resection, and use of the endoscope was associated with a slightly lower overall complication rate (78.2%). A similar rate of GTR (40%) has also been reported in patients with the endoscopic removal of tumors greater than 10 cm3.11 Compared to open and transsphenoidal microscopic surgery, respectively, EEA was associated with a significantly higher rate of improved visual outcomes (40% vs 34.8% vs 91.1%), lower recurrence rate (30% vs 20% vs 2.1%), and lower rate of hypopituitarism (9.1% vs 9.5% vs 1.06%).12
Craniopharyngiomas can be confined to the sella or can have significant suprasellar extension. The rates of GTR following EEA for craniopharyngiomas are, on average, 67%.13 In a recent systematic review of the literature, endoscopic resection of craniopharyngiomas yielded significantly greater rates of GTR than open surgery (66.9% vs 48.3%) and improved visual outcomes (56.2% and 33.1%). Recurrence rates were significantly lower in the transsphenoidal groups compared to open surgery. Although CSF leak rates were greater in the endoscopic (18.4%) and transsphenoidal microscopic (9%) groups compared with the open cohort (2.6%), more recent EEA series have reported rates of CSF leak of 3.8%.14 Seizures, although absent in the endonasal groups, occurred in 8.5% of patients undergoing open surgery. The rate of permanent diabetes insipidus was significantly greater in the transcranial cohort (54.8%) compared to those patients who underwent endoscopic (27.7%) and transsphenoidal microscopic surgery (31.7%), whereas the rate of panhypopituitarism was lowest in the latter group.13
Meningiomas are perhaps the most controversial tumors removed by endonasal endoscopic surgeons. This arises from the widely held belief, based on Simpson grading, that the goal of meningioma surgery is complete resection (i.e., Simpson grade 1), including the removal of all involved dura and bone,15 which some contend cannot be achieved via endonasal surgery.16 Recent literature, however, suggests that the Simpson grade may not be a significant predictor of recurrence-free survival for skull base tumors in the modern neurosurgical era.17 Nonetheless, advanced EEA techniques now permit more aggressive surgical removal of the planum sphenoidale, as well as opening of the medial optic canal to facilitate Simpson grade 1 results comparable to those achieved through a craniotomy.18
Results also vary based on the specific location of the meningioma. As recently demonstrated in a systematic review of the literature, the extent of resection is higher for planum and tuberculum tumors than for olfactory groove meningiomas, and thus the former location may be more suitable for EEA resection.19 Meningiomas can characteristically cause hyperostosis in adjacent bone, and, in many such cases, invade the bone, often serving as the primary site of recurrence for anterior skull base meningiomas. One advantage of the EEA is that removal of any infiltrated or hyperostotic bone at the cranial base is integral to the approach. Another distinct advantage is the ability to devascularize the tumor early in the operation by controlling the ethmoidal arteries, which usually constitute the main blood supply. This is often not possible until later in the resection using transcranial approaches.
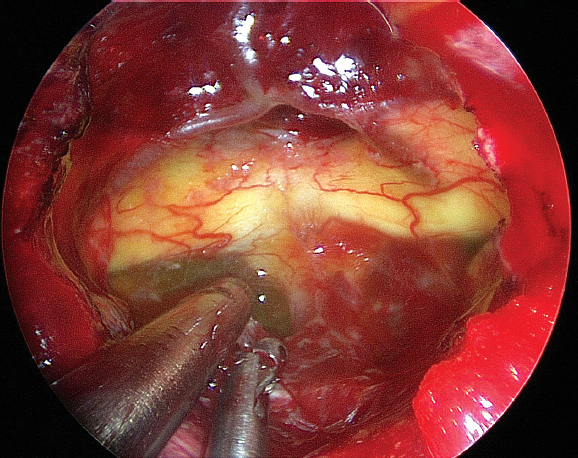
Perhaps the most significant advantage of the EEA for tuberculum and planum meningiomas is the ability to remove the tumor with minimal manipulation of the optic nerves. The optic nerve lies directly in the transcranial trajectory and is often extremely thinned (Fig. 13.2). The EEA affords a better view of the medial optic canal (assuming it is opened) than a transcranial approach. A recent review of the published literature revealed that visual improvement occurred more frequently (69.1%) following EEA surgery compared with transcranial surgery (58.7%).19 Tumor lateral to the optic nerve, however, cannot be removed endonasally. Although CSF leak rates reported in the literature for endonasal meningioma resection have been as high as 30%, more recent reports show that leak rates as low as 0% can be achieved with careful buttressed closure techniques.18,19
Pearl
• Extension of the transsphenoidal EEA through the tuberculum sella and planum sphenoidale permits resection of larger masses with suprasellar extension that lie between the carotid arteries with excellent results.
Transclival Approach
The clivus comprises the most ventral, midline posterior fossa skull base region, and thus surgical access can typically be quite difficult. Common neoplasms most commonly include chordomas, followed by chondrosarcomas, epidermoids/dermoids, and meningiomas. Various transfacial and transcranial approaches (i.e., subfrontal transbasal, anterior transfacial, subtemporal, transpetrosal or far lateral, transcondylar) have been described and can be used in combination during staged surgery.20,21 Although a midline ventral approach to the clivus makes the most anatomic sense, the transfacial approach is morbid and cosmetically disfiguring. Lateral or paramedian approaches, on the other hand, require the surgeon to traverse cranial nerves, as well as the vertebrobasilar arteries, to reach the clival-based tumor. Collective results of these open approaches vary widely, with a GTR rate ranging from 44 to 83%, neurological morbidity rates of 0 to 80%, and CSF leak rates of 8.3 to 30%.
The extended EEA to this region has many advantages, including the utilization of natural apertures and corridors and the ability to look laterally with angled endoscopes that decrease the manipulation of critical neurovascular structures. With careful case selection, the endonasal approaches to the clivus have shown excellent results compared with open approaches, particularly for chordomas and chondrosarcomas, which arise extradurally in a midline and paramedian location (Fig. 13.3). A report of 20 patients who underwent an EEA for primary and recurrent chordomas demonstrated the overall mean extent of resection to be 90.85%, with nearly half of the patients undergoing GTR.21 Extent of resection was significantly greater for primary chordomas (97.7%) compared with recurrent ones (81.8%). A consecutive case experience with seven patients who underwent EEA for clival chordomas similarly reported greater than 95% resection in 87% of patients, with greater extent of resection occurring in patients with smaller tumors (i.e., < 50 cm3).20
Transethmoidal Corridor
Malignant tumors involving the paranasal sinuses and anterior skull base, such as esthesioneuroblastoma and sinonasal undifferentiated carcinoma (SNUC), have long been treated with craniofacial surgery to achieve a goal of GTR with negative margins. Given the morbidity and often negative impact on quality of life, surgery has more recently been modified to a cranionasal approach with a combined EEA with bifrontal craniotomy when intracranial extension is evident (i.e., Kadish stage C). A purely EEA (without craniotomy), however, has become a reasonable alternative for achieving local control as long as the intracranial tumor does not extend lateral to the lamina papyracea.
A comparison of 120 patients with a wide variety of malignant sinonasal tumors who underwent a purely EEA (n = 93) versus cranionasal surgery (n = 27) showed that those patients with anterior skull base involvement and more extensive disease burden were more likely to undergo a combined cranionasal rather than purely endoscopic procedure.22 Disease survival and recurrence, however, were not significantly different between the two groups. Overall postoperative CSF leak was 3% in both groups. A recent meta-analysis compared these different surgical approaches for esthesioneuroblastoma and found GTR was achieved in 98.1% after EEA surgery, compared to 81.3% of patients undergoing craniofacial resection. All patients undergoing cranionasal resection experienced GTR.23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree